Home /
Expert Answers /
Chemistry /
consider-the-reaction-2h-g-o2-g-2ho-g-using-standard-thermodynamic-data-at-298-k-pa383
(Solved): Consider the reaction: 2H(g) + O2(g) 2HO(g) Using standard thermodynamic data at 298 K, ...
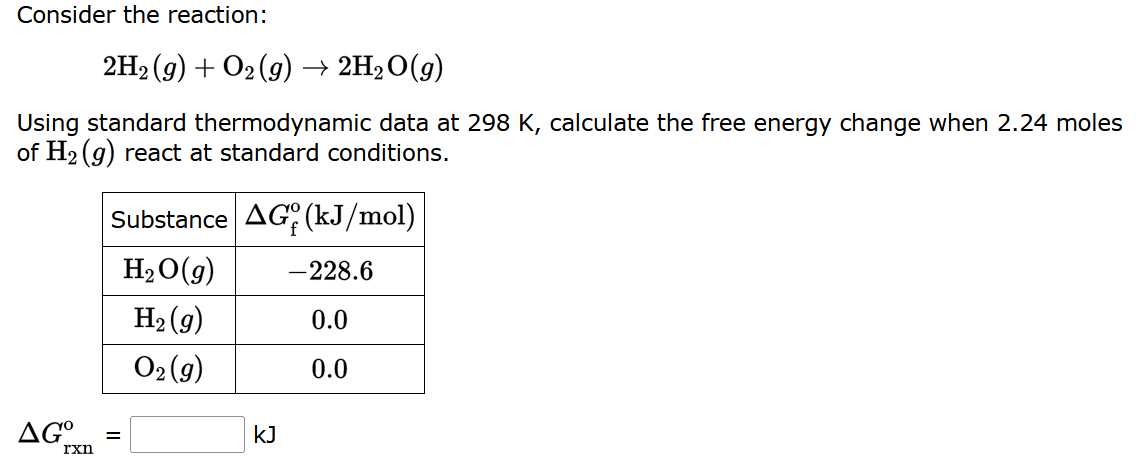
Consider the reaction: 2H?(g) + O2(g) ? 2H?O(g) Using standard thermodynamic data at 298 K, calculate the free energy change when 2.24 moles of H?(g) react at standard conditions. AGO rxn Substance AG (kJ/mol) H?O(g) H?(g) 02 (9) = KJ -228.6 0.0 0.0
Expert Answer
Solution: ?Grxn=?512.06 kJ Explanation: Step 1- Write the balanced equation. 2H2+O2?2H2O Step 2- Calculate the free energy of the reaction. Calculate the free energy of the reac