Home /
Expert Answers /
Chemical Engineering /
consider-the-phase-diagram-of-the-gold-lead-au-pb-system-a-indicate-the-phase-s-present-in-regio-pa332
(Solved): Consider the phase diagram of the gold-lead (Au-Pb) system.a) Indicate the phase(s) present in regio ...
Consider the phase diagram of the gold-lead (Au-Pb) system.
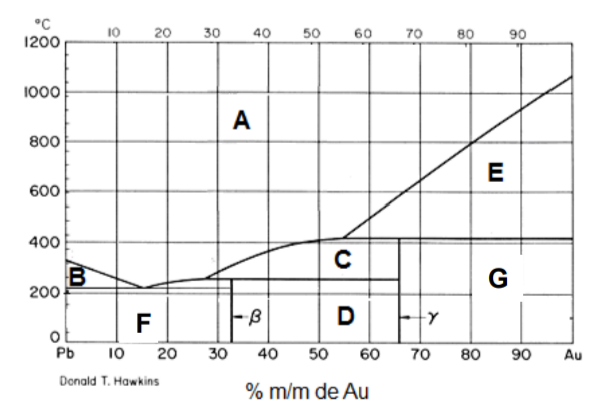
a) Indicate the phase(s) present in regions A, B, C, D, E, F and G.
b) Indicate the number of degrees of freedom of a system located in regions A, D and E.
c) Determine the minimum formulas of the compounds y and ? formed, knowing that the mass molar of gold is 197.0 g mol-1 and that of lead is 207.2 mol-1 .
d) Determine the melting temperature of the y phase and the peritectic composition present in the transition e) Estimate the melting temperature and composition of the eutectic mixture.