Home /
Expert Answers /
Chemistry /
consider-the-phase-diagram-for-water-shown-in-the-figure-in-what-pressure-range-and-in-w-pa653
Expert Answer
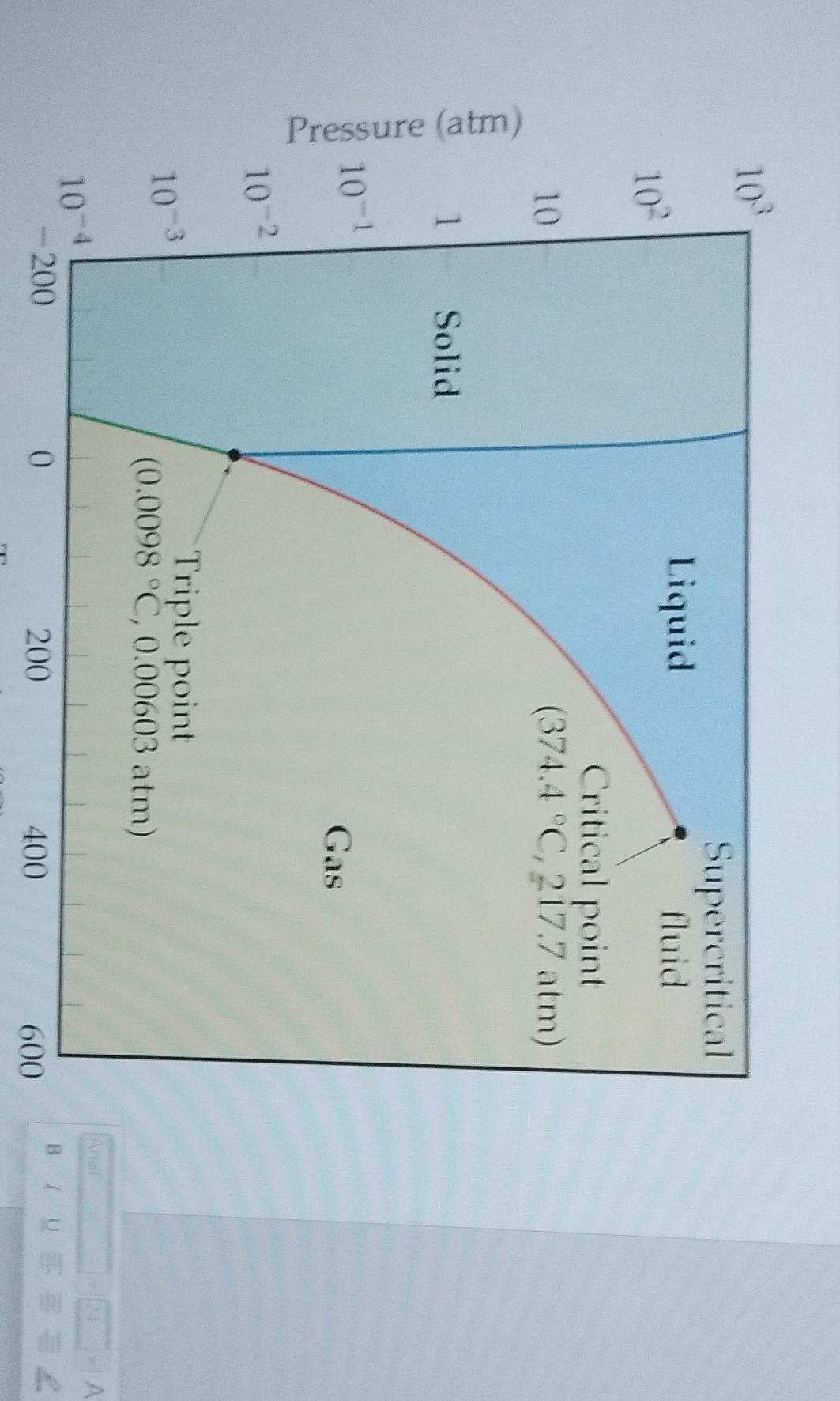
To determine the pressure range and temperature range in which H2O(s) can sublime to H2O(g) based on the given phase diagram, we need to consider the conditions at which sublimation occurs.Sublimation is the process in which a substance transitions directly from a solid phase to a gas phase without going through the liquid phase. In the phase diagram, this transition occurs along the sublimation curve, which separates the solid and gas phases.Based on the phase diagram provided, let's analyze the different regions: