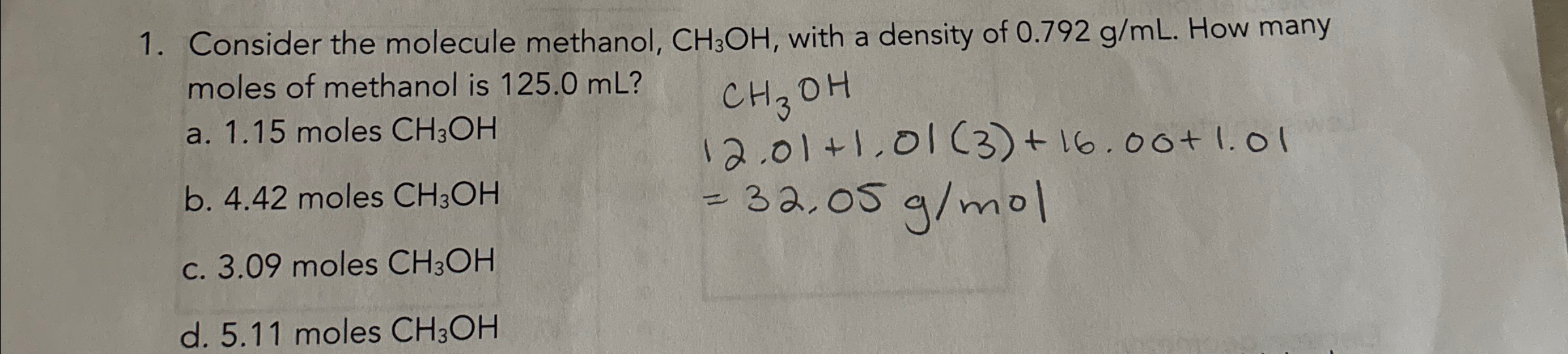Home /
Expert Answers /
Chemistry /
consider-the-molecule-methanol-ch-3-oh-with-a-density-of-0-792-g-m-l-how-many-moles-of-methano-pa756
(Solved): Consider the molecule methanol, CH_(3)OH, with a density of 0.792(g)/(m)L. How many moles of methano ...
Consider the molecule methanol,
CH_(3)OH, with a density of
0.792(g)/(m)L. How many moles of methanol is
125.0mL? a. 1.15 moles
CH_(3)OHb. 4.42 moles
CH_(3)OHc. 3.09 moles
CH_(3)OHd. 5.11 moles
CH_(3)OH