Home /
Expert Answers /
Chemistry /
consider-the-following-unbalanced-particulate-representation-of-a-chemical-equation-n-blue-o-pa145
(Solved): Consider the following unbalanced particulate representation of a chemical equation: N = blue O = ...
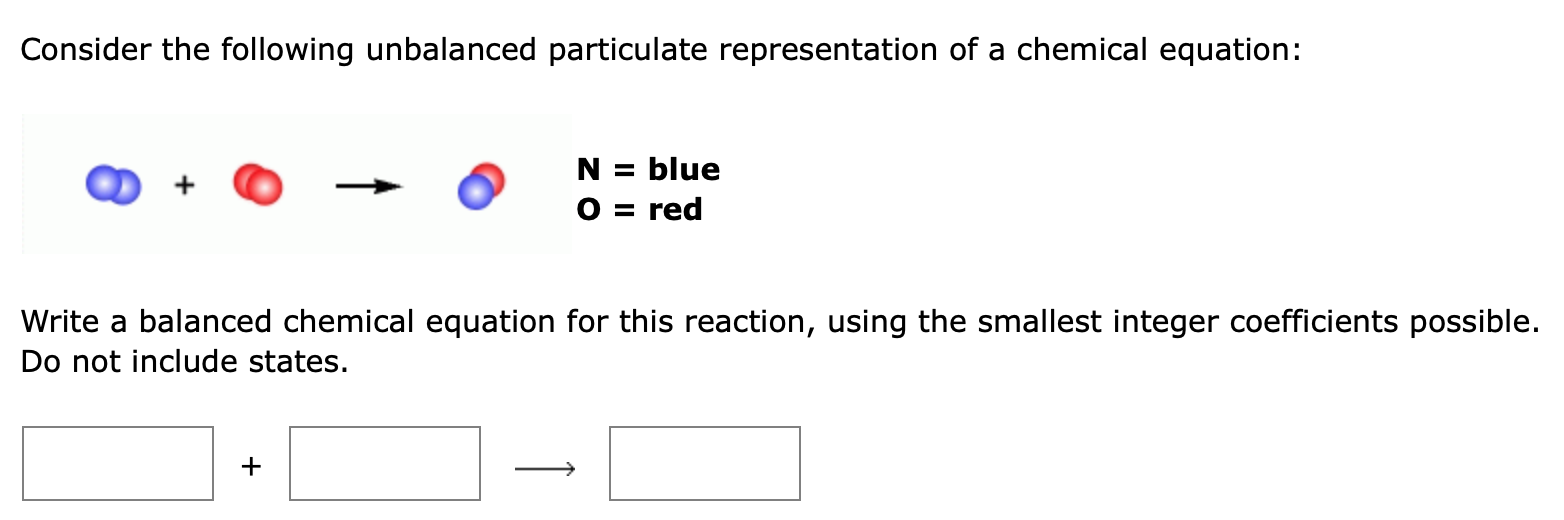
Consider the following unbalanced particulate representation of a chemical equation: N = blue O = red Write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Do not include states. +
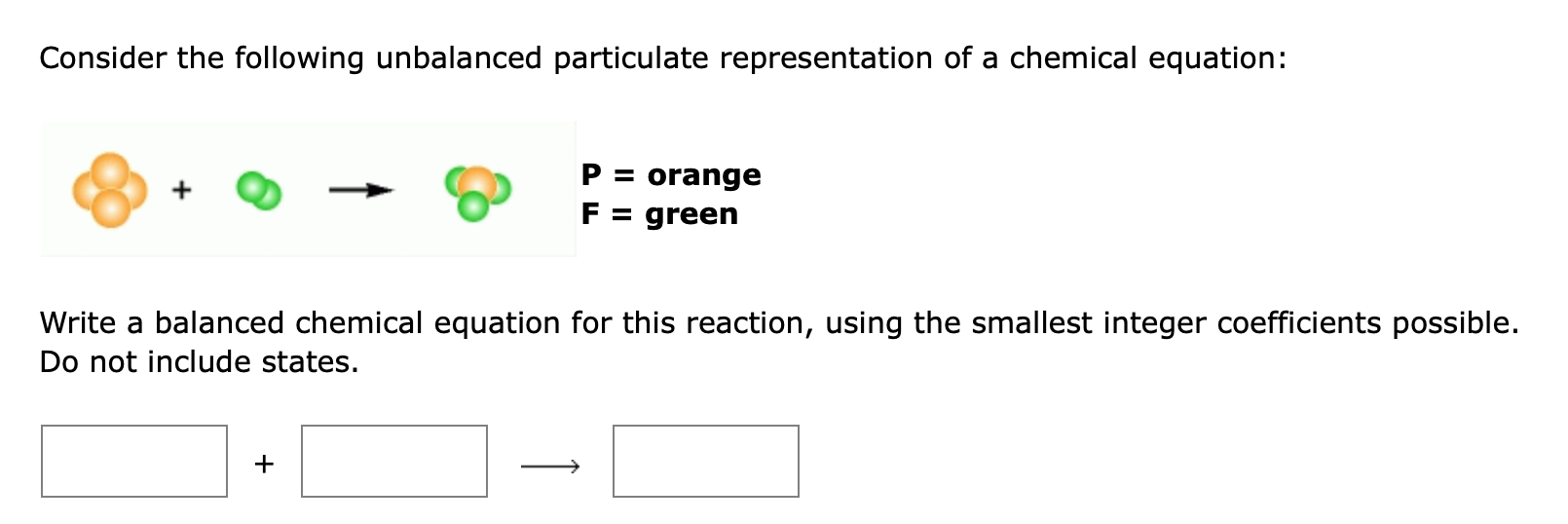
Consider the following unbalanced particulate representation of a chemical equation: P = orange + F = green Write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Do not include states. +
Consider the following unbalanced particulate representation of a chemical equation: Xe = brown F: = green Write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Do not include states. +
Expert Answer
Ans- 1- As we can see from the above question blue = nitrogen and red = oxygen 2blue + 2red (blue + red) N2 + 02 NO To balance the chemical reaction we have to e