Home /
Expert Answers /
Chemistry /
consider-the-following-unbalanced-particulate-representation-of-a-chemical-equation-b1-qi-pa935
(Solved): Consider the following unbalanced particulate representation of a chemical equation: (B1)+QI ...
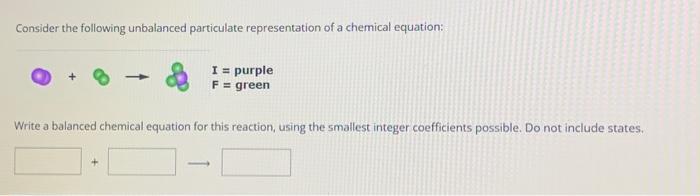
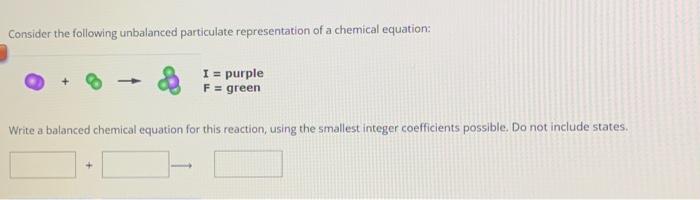
Consider the following unbalanced particulate representation of a chemical equation: Write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Do not include states.
Consider the following unbalanced particulate representation of a chemical equation: Write a balanced chemical equation for this reaction, using the smallest integer coefficients possible. Do not include states.
Expert Answer
The balanced chemical equation for the reaction between iodine and fluorine to form iodine trifluoride is: