Home /
Expert Answers /
Chemistry /
consider-the-following-two-half-reactions-and-their-standard-reduction-potentials-mathrm-o-pa950
(Solved): Consider the following two half-reactions and their standard reduction potentials. \[ \mathrm{O}_{ ...
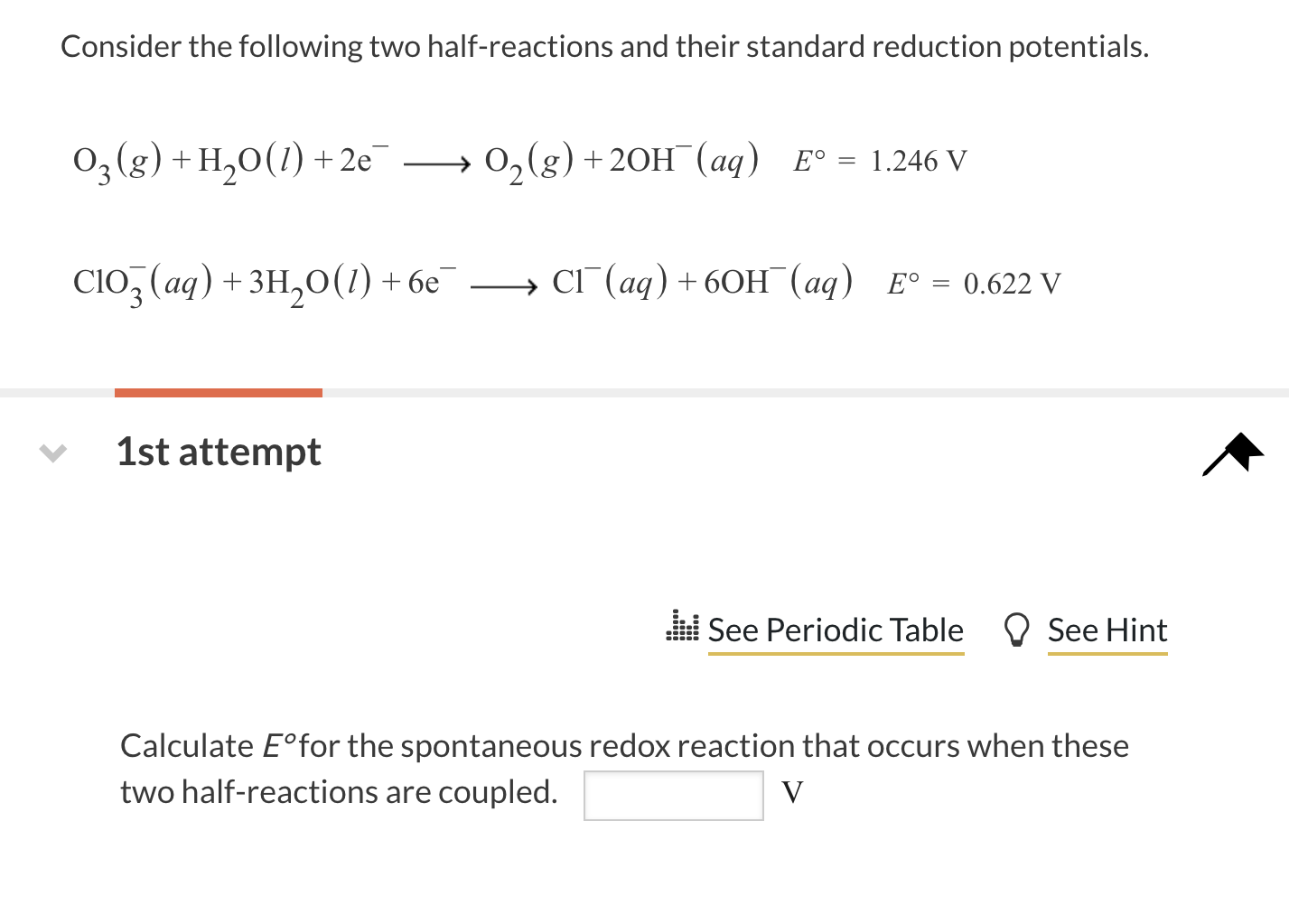
Consider the following two half-reactions and their standard reduction potentials. \[ \mathrm{O}_{3}(g)+\mathrm{H}_{2} \mathrm{O}(l)+2 \mathrm{e}^{-} \longrightarrow \mathrm{O}_{2}(g)+2 \mathrm{OH}^{-}(a q) \quad E^{\circ}=1.246 \mathrm{~V} \] \[ \mathrm{ClO}_{3}^{-}(a q)+3 \mathrm{H}_{2} \mathrm{O}(l)+6 \mathrm{e}^{-} \longrightarrow \mathrm{Cl}^{-}(a q)+6 \mathrm{OH}^{-}(a q) \quad E^{\circ}=0.622 \mathrm{~V} \] 1st attempt 0 Calculate \( E^{\circ} \) for the spontaneous redox reaction that occurs when these two half-reactions are coupled. \( \mathrm{V} \)