Home /
Expert Answers /
Chemistry /
consider-the-following-reaction-for-the-decomposition-of-hydrogen-disulfide-2h2s-g-lt-pa839
(Solved): Consider the following reaction for the decomposition of hydrogen disulfide: 2H2S(g)<= ...
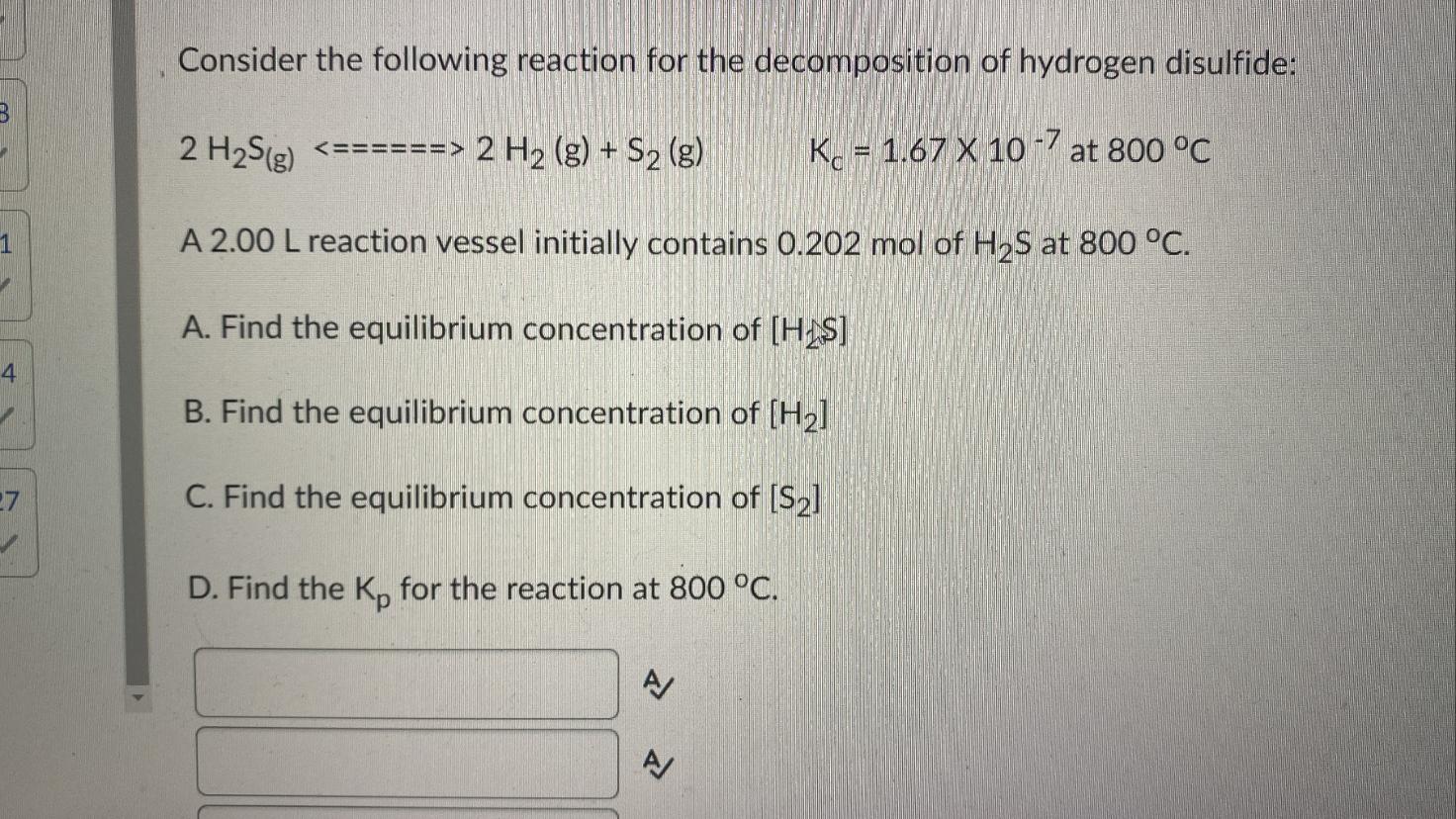
Consider the following reaction for the decomposition of hydrogen disulfide: A 2.00 L reaction vessel initially contains of at . A. Find the equilibrium concentration of B. Find the equilibrium concentration of C. Find the equilibrium concentration of D. Find the for the reaction at . A A