Home /
Expert Answers /
Chemistry /
consider-the-following-reaction-cocl2-g-co-g-cl2-g-at25c-a-reaction-mixture-pa259
(Solved): Consider the following reaction: COCl2(g)=CO(g)+Cl2(g)at25C A reaction mixture ...
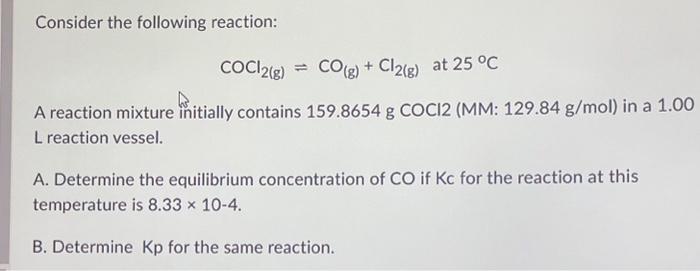
Consider the following reaction: A reaction mixture initially contains (MM: ) in a 1.00 reaction vessel. A. Determine the equilibrium concentration of if for the reaction at this temperature is B. Determine for the same reaction.