Home /
Expert Answers /
Chemistry /
consider-the-following-reaction-between-mercury-ii-chlonide-and-oxalate-lon-2-mathrm-hgcl-2-pa520
(Solved): Consider the following reaction between mercury(II) chlonide and oxalate lon. \[ 2 \mathrm{HgCl}_{2 ...
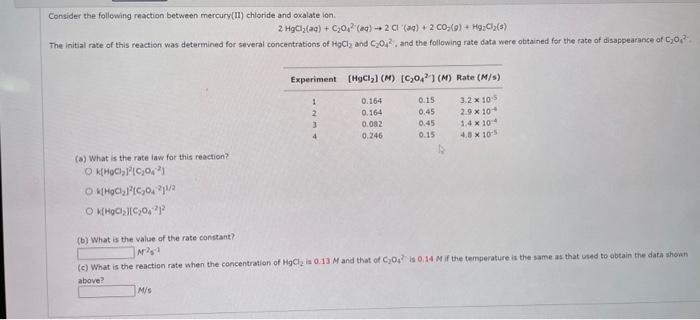
Consider the following reaction between mercury(II) chlonide and oxalate lon. \[ 2 \mathrm{HgCl}_{2}(\mathrm{aq})+\mathrm{C}_{2} \mathrm{O}_{4}^{2} \text { (aq) } \rightarrow 2 \mathrm{Cl}^{-}(\mathrm{aq})+2 \mathrm{CO}_{2}(9)+\mathrm{Hg}_{2} \mathrm{Cl}_{2}(s) \] The initial rate of this reaction was determined for several concentrations of \( \mathrm{HgCl}_{2} \) and \( \mathrm{C}_{2} \mathrm{O}_{4}^{2} \), and the following rate data were obtained for the fate of disappearance of \( \mathrm{C}_{2} \mathrm{O}_{4}^{2} \). (a) What is the rate law for this reaction? \( \mathrm{k}\left[\mathrm{HOCl}_{2}\right]^{2}\left[\mathrm{C}_{2} \mathrm{O}_{4}{ }^{-2}\right] \) \( \mathrm{W}\left(\mathrm{HgCl}_{2}\right)^{2}\left(\mathrm{C}_{2} \mathrm{O}_{4}{ }^{2} \mathrm{~J}^{\mathrm{H} / 2}\right. \) k[ \( \left.\mathrm{HgC}_{2}\right]\left[\mathrm{C}_{2} \mathrm{O}_{4}{ }^{-2}\right]^{2} \) (b) What is the value of the rate constant? \[ \mathrm{N}^{2} \mathrm{~s}^{-1} \] (c) What is the ceaction rate when the concentration of \( \mathrm{HgCl}_{2} \) is \( 0.13 \mathrm{M} \) and that of \( \mathrm{C}_{2} \mathrm{O}_{4}^{2} \) is \( 0,14 \mathrm{M} \) if the temperature is the same as that ised to abtain the data shionh above?
Expert Answer
Please upvote if the solu