Home /
Expert Answers /
Chemistry /
consider-the-following-exothermic-e2-reaction-what-rate-equation-would-be-observed-for-this-reactio-pa449
(Solved): Consider the following exothermic E2 reaction. What rate equation would be observed for this reactio ...
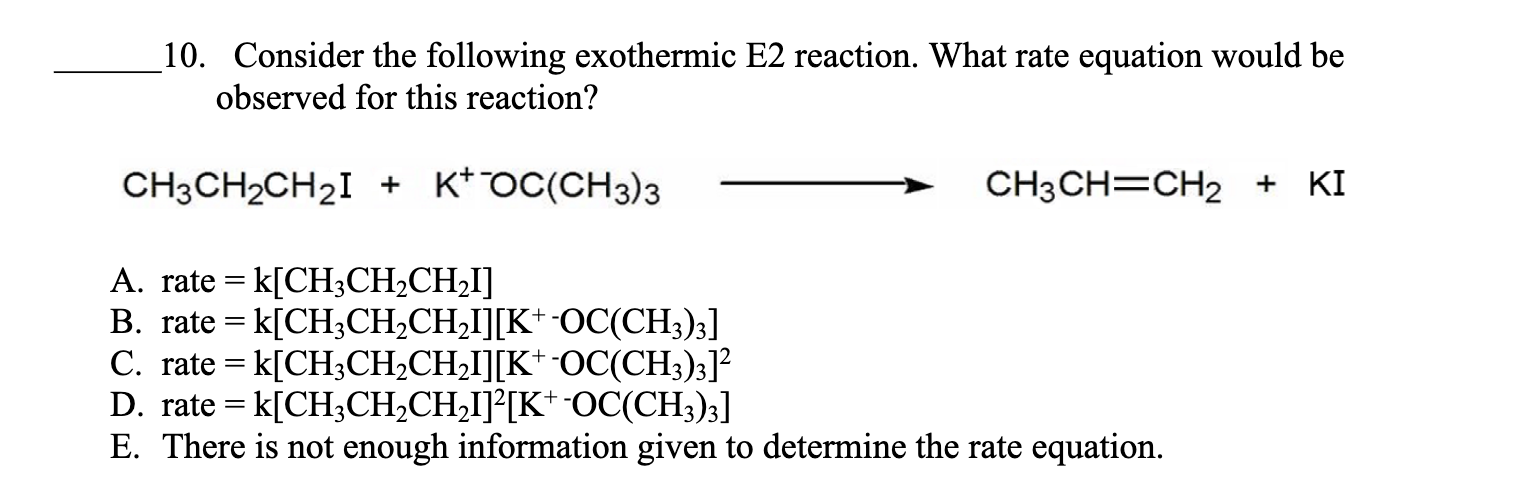
Consider the following exothermic E2 reaction. What rate equation would be
observed for this reaction?
CH_(3)CH_(2)CH_(2)I+K^(+-)OC(CH_(3))_(3)longrightarrowCH_(3)CH=CH_(2)+KI
A. rate =k[CH_(3)CH_(2)CH_(2)I]
B. rate =k[CH_(3)CH_(2)CH_(2)I][K^(+)-OC(CH_(3))_(3)]
C. rate =k[CH_(3)CH_(2)CH_(2)I][K^(+)-OC(CH_(3))_(3)]^(2)
D. rate =k[CH_(3)CH_(2)CH_(2)I]^(2)[K^(+-)OC(CH_(3))_(3)]
E. There is not enough information given to determine the rate equation.