Home /
Expert Answers /
Chemistry /
consider-the-following-chemical-reaction-nh3-g-2-o-g-hno3-aq-ho-1-calculate-the-pa903
(Solved): Consider the following chemical reaction. NH3(g) + 2 O(g) HNO3(aq) + HO(1) Calculate the ...
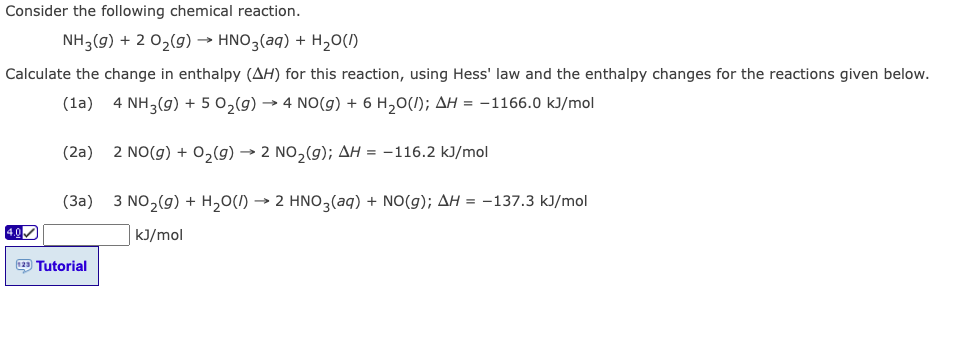
Consider the following chemical reaction. NH3(g) + 2 O?(g) ? HNO3(aq) + H?O(1) Calculate the change in enthalpy (AH) for this reaction, using Hess' law and the enthalpy changes for the reactions given below. (1a) 4 NH3(g) + 5 O?(g) ? 4 NO(g) + 6 H?O(); AH = -1166.0 kJ/mol 4.0? (2a) (3a) 123 Tutorial 2 NO(g) + O?(g) ? 2 NO?(g); AH = -116.2 kJ/mol 3 NO?(g) + H?O() ? 2 HNO3(aq) + NO(g); AH = -137.3 kJ/mol kJ/mol