Home /
Expert Answers /
Chemistry /
consider-the-following-balanced-equation-for-the-combustion-of-butane-a-fuel-often-used-in-lighter-pa683
(Solved): Consider the following balanced equation for the combustion of butane, a fuel often used in lighter ...
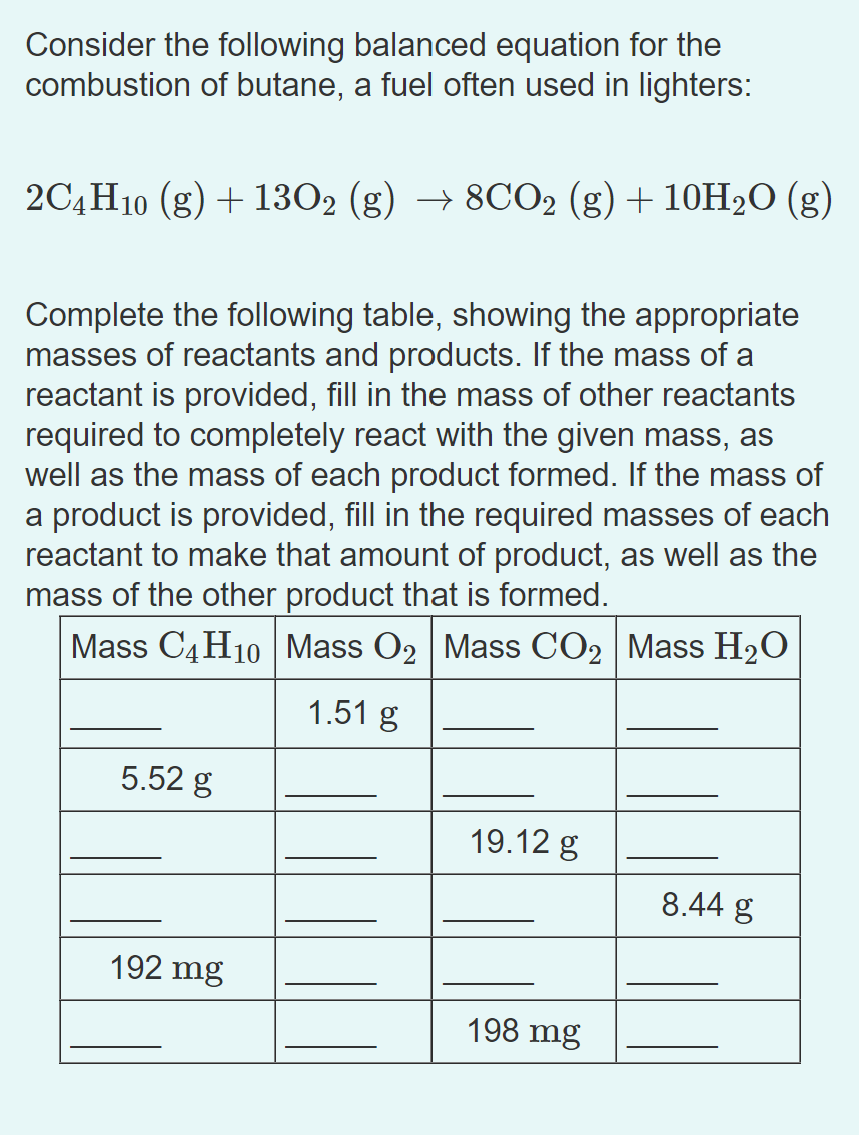
Consider the following balanced equation for the combustion of butane, a fuel often used in lighters: Complete the following table, showing the appropriate masses of reactants and products. If the mass of a reactant is provided, fill in the mass of other reactants required to completely react with the given mass, as well as the mass of each product formed. If the mass of a product is provided, fill in the required masses of each reactant to make that amount of product, as well as the mass of the other product that is formed.