Home /
Expert Answers /
Chemistry /
consider-the-following-balanced-chemical-reaction-b2o3-g-3-caf-aq-2-bf3-g-3-cao-s-pa679
(Solved): Consider the following balanced chemical reaction: B2O3(g) + 3 CaF(aq) 2 BF3(g) + 3 CaO(s) + ...
Consider the following balanced chemical reaction: B2O3(g) + 3 CaF?(aq) ? 2 BF3(g) + 3 CaO(s) + AH 752.33 kJ = a.) Is the reaction exothermic or endothermic? b.) How much heat, in Joules, is released or absorbed to fully react 275 mL of 6.00 M calcium fluoride with excess B?O3? c.) What could be the possible theoretical yield of CaO, in moles, when 1.25x104 Joules of heat is released or absorbed by the reaction?
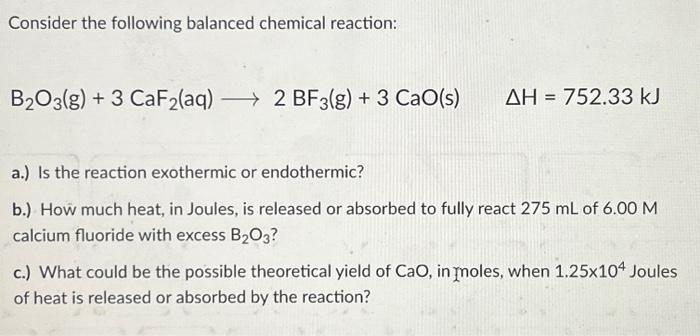
Consider the following balanced chemical reaction: a.) Is the reaction exothermic or endothermic? b.) How much heat, in Joules, is released or absorbed to fully react of calcium fluoride with excess ? c.) What could be the possible theoretical yield of , in ynoles, when Joules of heat is released or absorbed by the reaction?
Expert Answer
a.) Is the reaction exothermic or endothermic?The reaction is exothermic. This is because the heat ...