Home /
Expert Answers /
Chemistry /
consider-the-equilibrium-system-described-by-the-chemical-reaction-below-determine-the-concentrati-pa769
(Solved): Consider the equilibrium system described by the chemical reaction below. Determine the concentrati ...
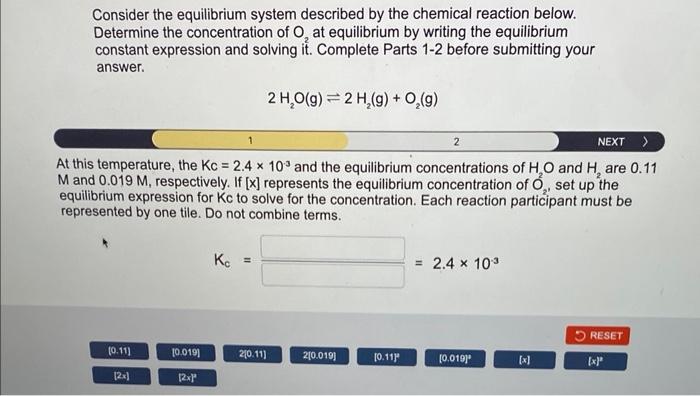
Consider the equilibrium system described by the chemical reaction below. Determine the concentration of \( \mathrm{O}_{2} \) at equilibrium by writing the equilibrium constant expression and solving it. Complete Parts 1-2 before submitting your answer. \[ 2 \mathrm{H}_{2} \mathrm{O}(\mathrm{g}) \rightleftharpoons 2 \mathrm{H}_{2}(\mathrm{~g})+\mathrm{O}_{2}(\mathrm{~g}) \] th this temperature, the \( \mathrm{Kc}=2.4 \times 10^{-3} \) and the equilibrium concentrations of \( \mathrm{H}_{2} \mathrm{O} \) and \( \mathrm{H}_{2} \) are \( 0.11 \) \( A \) and \( 0.019 \mathrm{M} \), respectively. If \( [x] \) represents the equilibrium concentration of \( \mathrm{O}_{2} \), set up the quilibrium expression for Kc to solve for the concentration. Each reaction participant must be epresented by one tile. Do not combine terms. \[ \mathrm{K}_{\mathrm{c}}=\quad=2.4 \times 10^{-3} \]