Home /
Expert Answers /
Chemistry /
consider-the-electrochemical-cell-diagram-shown-below-as-you-observe-the-reaction-in-the-cell-you-pa984
(Solved): Consider the electrochemical cell diagram shown below. As you observe the reaction in the cell, you ...
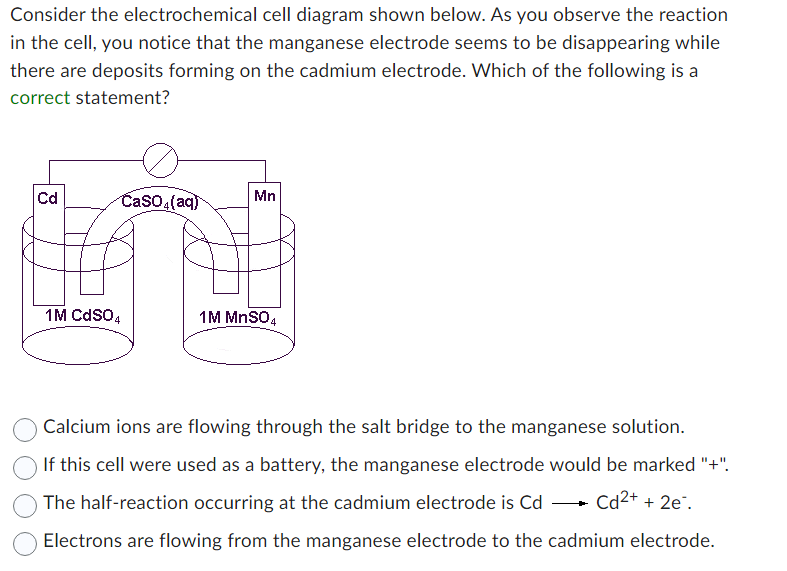
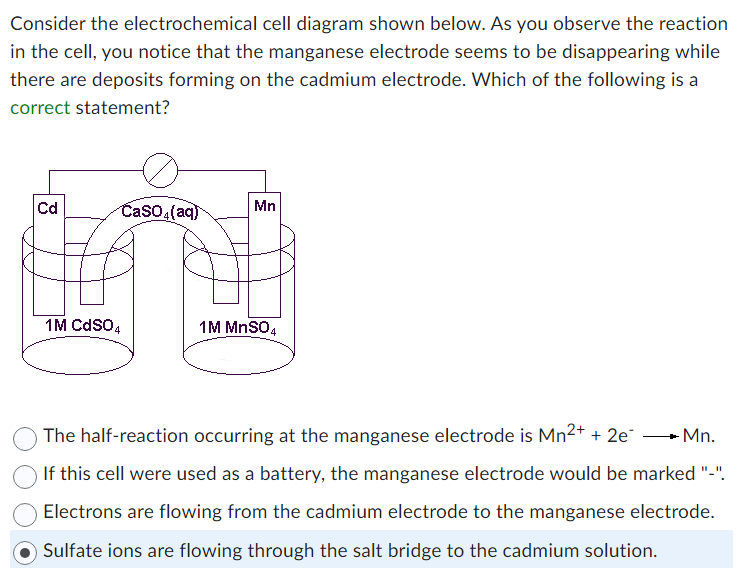
Consider the electrochemical cell diagram shown below. As you observe the reaction in the cell, you notice that the manganese electrode seems to be disappearing while there are deposits forming on the cadmium electrode. Which of the following is a correct statement? Calcium ions are flowing through the salt bridge to the manganese solution. If this cell were used as a battery, the manganese electrode would be marked \"+\". The half-reaction occurring at the cadmium electrode is \\( \\mathrm{Cd} \\longrightarrow \\mathrm{Cd}^{2+}+2 \\mathrm{e}^{-} \\). Electrons are flowing from the manganese electrode to the cadmium electrode.\r\n\r\nConsider the electrochemical cell diagram shown below. As you observe the reaction in the cell, you notice that the manganese electrode seems to be disappearing while there are deposits forming on the cadmium electrode. Which of the following is a correct statement? The half-reaction occurring at the manganese electrode is \\( \\mathrm{Mn}^{2+}+2 \\mathrm{e}^{-} \\longrightarrow \\mathrm{Mn} \\). If this cell were used as a battery, the manganese electrode would be marked \"-\". Electrons are flowing from the cadmium electrode to the manganese electrode. Sulfate ions are flowing through the salt bridge to the cadmium solution.
Expert Answer
The correct statement is: Electrons are flowing from the manganese electrode to the cadmium electrod...