Home /
Expert Answers /
Chemistry /
consider-the-decomposition-of-calcium-carbonate-caco3-s-cao-s-co2-g-h-177-8molkj-pa466
(Solved): Consider the decomposition of calcium carbonate: CaCO3(s)CaO(s)+CO2(g)H=177.8molkJ ...
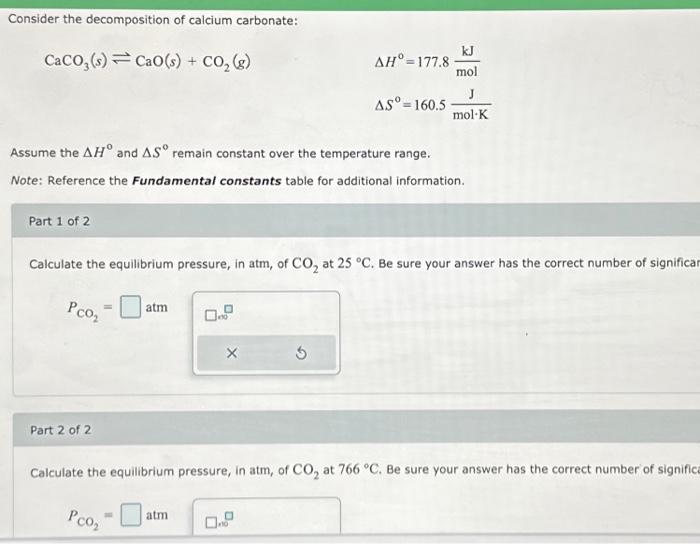
Consider the decomposition of calcium carbonate: Assume the and remain constant over the temperature range. Note: Reference the Fundamental constants table for additional information. Part 1 of 2 Calculate the equilibrium pressure, in atm, of at . Be sure your answer has the correct number of significa Part 2 of 2 Calculate the equilibrium pressure, in atm, of at . Be sure your answer has the correct number of signific
Expert Answer
The main objective of this question is to calculate the equilibrium pressure of at different temp...