Home /
Expert Answers /
Chemistry /
consider-the-combustion-of-liquid-c5h8-in-oxygen-gas-to-produce-carbon-dioxide-gas-and-water-pa983
(Solved): Consider the combustion of liquid C5H8 in oxygen gas to produce carbon dioxide gas and water ...
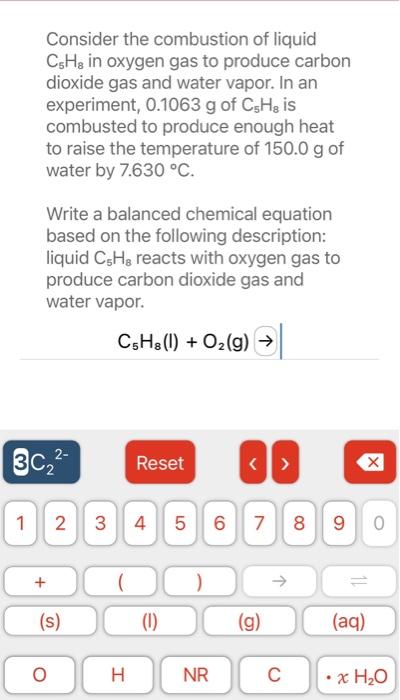
Consider the combustion of liquid in oxygen gas to produce carbon dioxide gas and water vapor. In an experiment, of is combusted to produce enough heat to raise the temperature of of water by . Write a balanced chemical equation based on the following description: liquid reacts with oxygen gas to produce carbon dioxide gas and water vapor.
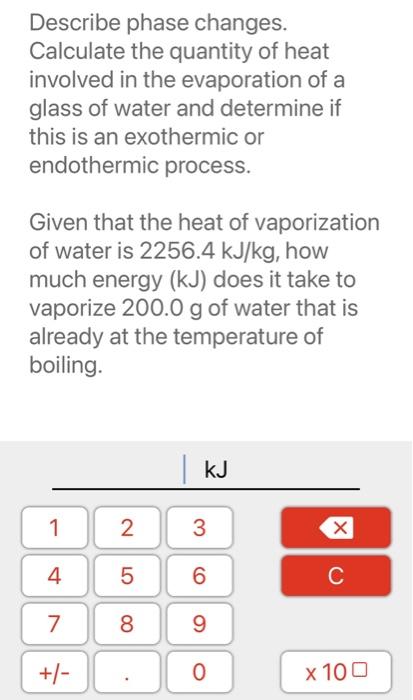
Describe phase changes. Calculate the quantity of heat involved in the evaporation of a glass of water and determine if this is an exothermic or endothermic process. Given that the heat of vaporization of water is , how much energy ( ) does it take to vaporize of water that is already at the temperature of boiling.