Home /
Expert Answers /
Chemistry /
consider-the-buffer-system-of-acetic-acid-mathrm-hc-2-mathrm-h-3-mathrm-o-2-and-pa160
(Solved): Consider the buffer system of acetic acid, \( \mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2} \), and ...
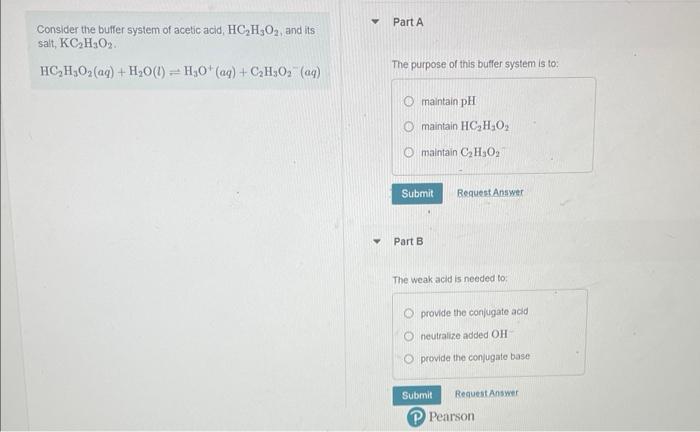
Consider the buffer system of acetic acid, \( \mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2} \), and its salt, \( \mathrm{KC}_{2} \mathrm{H}_{3} \mathrm{O}_{2} \). \( \mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q)+\mathrm{H}_{2} \mathrm{O}(l) \rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{C}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q) \) The purpose of this buffer system is to: maintain \( \mathrm{pH} \) maintain \( \mathrm{HC}_{2} \mathrm{H}_{2} \mathrm{O}_{2} \) maintain \( \mathrm{C}_{2} \mathrm{H}_{3} \mathrm{O}_{2} \) Part B The weak acid is needed to: provide the conjugate acid neutralize added \( \mathrm{OH}^{-} \) provide the conlugate base
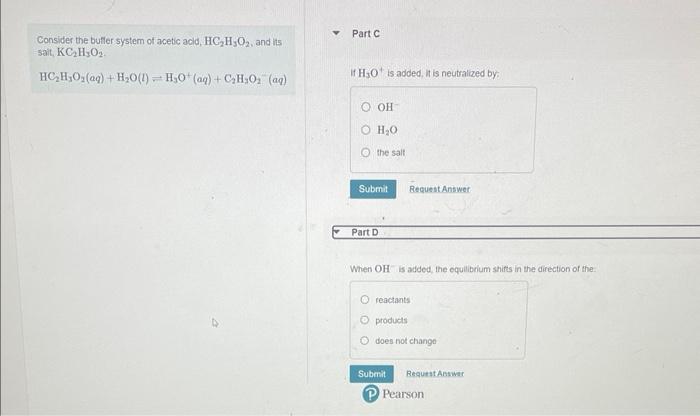
Consider the buffer system of acetic acld, \( \mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2} \), and its sait, \( \mathrm{KC}_{2} \mathrm{H}_{3} \mathrm{O}_{2} \). \( \mathrm{HC}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q)+\mathrm{H}_{2} \mathrm{O}(l) \rightleftharpoons \mathrm{H}_{3} \mathrm{O}^{+}(a q)+\mathrm{C}_{2} \mathrm{H}_{3} \mathrm{O}_{2}(a q) \) If \( \mathrm{H}_{3} \mathrm{O}^{+} \)is added, it is neutralized by: When \( \mathrm{OH} \) is added, the equilbrium shifts in the direction of the. reactants products does not chango
Expert Answer
Given a buffer system of acetic acid, HC2H3O2 and it's salt KC2H3O2. Given reaction - HC2H3O2(aq) + H2O(l) H3O+(aq