Home /
Expert Answers /
Chemistry /
consider-hte-following-unbalanced-chemical-equation-mathrm-c-6-mathrm-h-14-mathrm-o-pa904
(Solved): Consider hte following unbalanced chemical equation: \[ -\mathrm{C}_{6} \mathrm{H}_{14}+\mathrm{O}_ ...
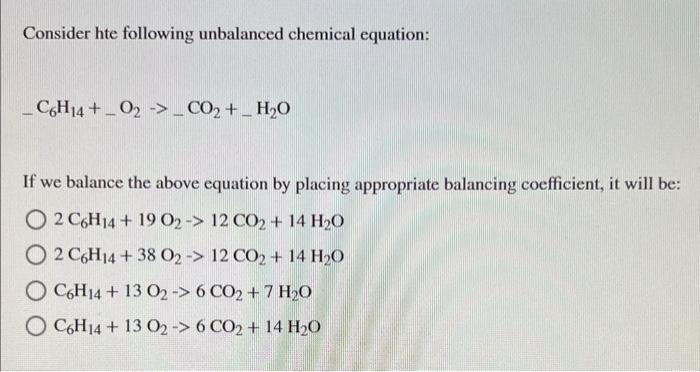
Consider hte following unbalanced chemical equation: \[ -\mathrm{C}_{6} \mathrm{H}_{14}+\mathrm{O}_{2}-\mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O} \] If we balance the above equation by placing appropriate balancing coefficient, it will be: \[ \begin{array}{l} 2 \mathrm{C}_{6} \mathrm{H}_{14}+19 \mathrm{O}_{2} \rightarrow 12 \mathrm{CO}_{2}+14 \mathrm{H}_{2} \mathrm{O} \\ 2 \mathrm{C}_{6} \mathrm{H}_{14}+38 \mathrm{O}_{2} \rightarrow 12 \mathrm{CO}_{2}+14 \mathrm{H}_{2} \mathrm{O} \\ \mathrm{C}_{6} \mathrm{H}_{14}+13 \mathrm{O}_{2} \rightarrow 6 \mathrm{CO}_{2}+7 \mathrm{H}_{2} \mathrm{O} \\ \mathrm{C}_{6} \mathrm{H}_{14}+13 \mathrm{O}_{2} \rightarrow 6 \mathrm{CO}_{2}+14 \mathrm{H}_{2} \mathrm{O} \end{array} \]