Home /
Expert Answers /
Chemistry /
consider-an-ideal-gas-enclosed-in-a-1-00l-container-at-an-internal-pressure-of-18-0atm-calcula-pa487
(Solved): Consider an ideal gas enclosed in a 1.00L container at an internal pressure of 18.0atm. Calcula ...
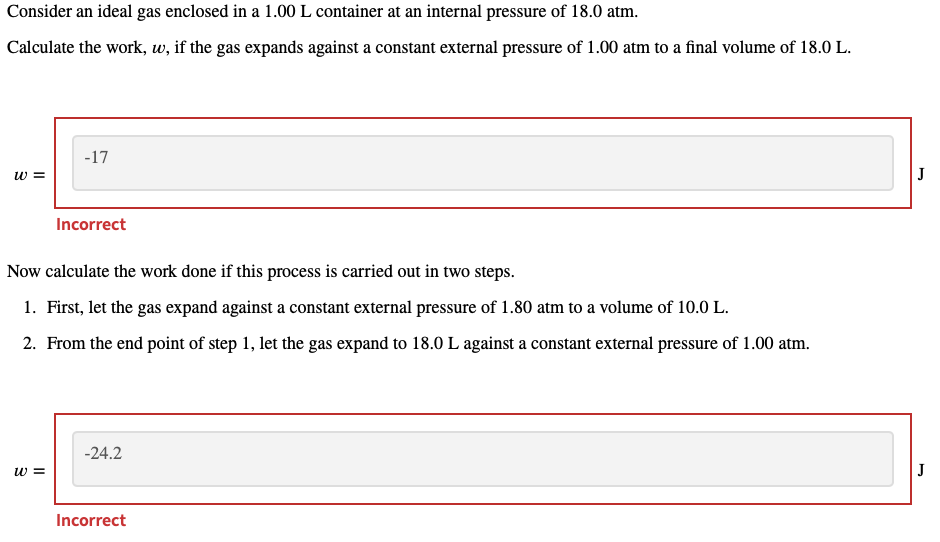
Consider an ideal gas enclosed in a container at an internal pressure of . Calculate the work, , if the gas expands against a constant external pressure of to a final volume of . Incorrect Now calculate the work done if this process is carried out in two steps. 1. First, let the gas expand against a constant external pressure of to a volume of . 2. From the end point of step 1, let the gas expand to against a constant external pressure of .
Expert Answer
Given: Internal pressure (Pi) = 18.0 atm External pressure (Pext) = 1.00 atm Initial volume (Vi) = 1...