Home /
Expert Answers /
Chemistry /
consider-a-transition-of-the-electron-in-the-hydrogen-atom-from-n-3-to-n-7-is-del-pa419
(Solved): Consider a transition of the electron in the hydrogen atom from \( n=3 \) to \( n=7 \). Is \( \Del ...
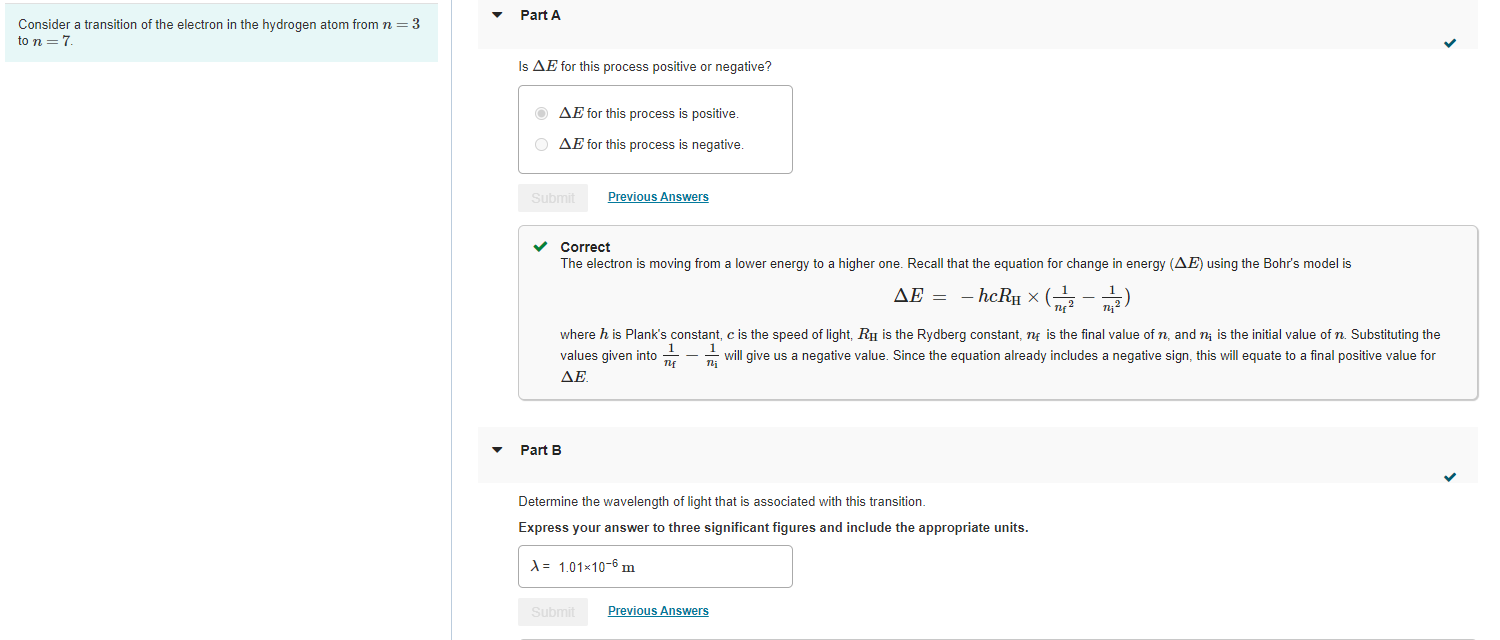
Consider a transition of the electron in the hydrogen atom from \( n=3 \) to \( n=7 \). Is \( \Delta E \) for this process positive or negative? \( \Delta E \) for this process is positive. \( \Delta E \) for this process is negative. Correct The electron is moving from a lower energy to a higher one. Recall that the equation for change in energy \( (\Delta E) \) using the Bohr's model is \[ \Delta E=-h c R_{\mathrm{H}} \times\left(\frac{1}{n_{\mathrm{f}}{ }^{2}}-\frac{1}{n_{\mathrm{i}}{ }^{2}}\right) \] where \( h \) is Plank's constant, \( c \) is the speed of light, \( R_{\mathrm{H}} \) is the Rydberg constant, \( n_{\mathrm{f}} \) is the final value of \( n \), and \( n_{\mathrm{i}} \) is the initial value of \( n \). Substituting the values given into \( \frac{1}{n_{\mathrm{f}}}-\frac{1}{n_{\mathrm{i}}} \) will give us a negative value. Since the equation already includes a negative sign, this will equate to a final positive value for \( \Delta E \). - Part B Determine the wavelength of light that is associated with this transition. Express your answer to three significant figures and include the appropriate units.
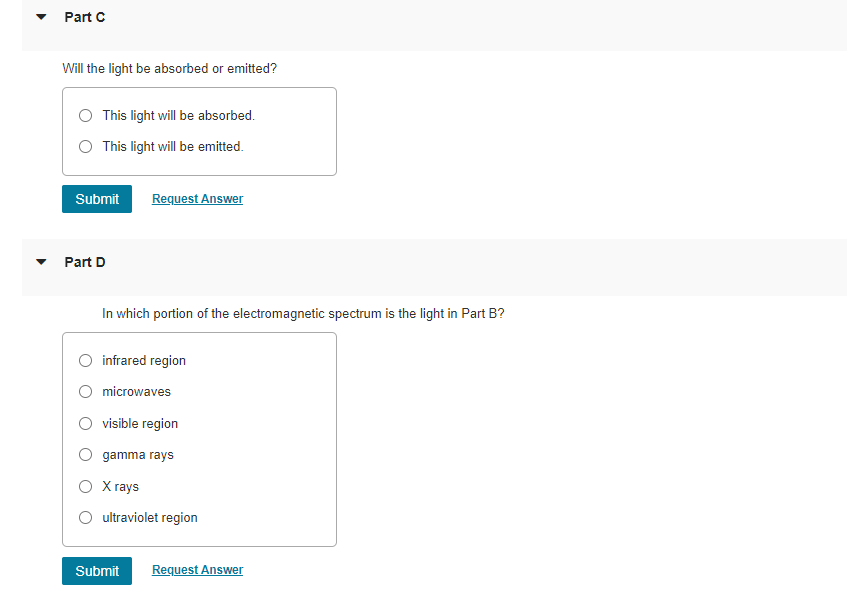
Will the light be absorbed or emitted? This light will be absorbed. This light will be emitted. Part D In which portion of the electromagnetic spectrum is the light in Part B? infrared region microwaves visible region gamma rays \( X \) rays ultraviolet region