Home /
Expert Answers /
Chemistry /
compound-21-sef-22-becl-lewis-dot-structure-ti-ti-se-t-inf-central-atom-se-steric-numbe-pa311
(Solved): Compound 21. Sef 22. BeCl Lewis Dot Structure TI: TI ( Se :T!: INF: Central Atom Se Steric Numbe ...
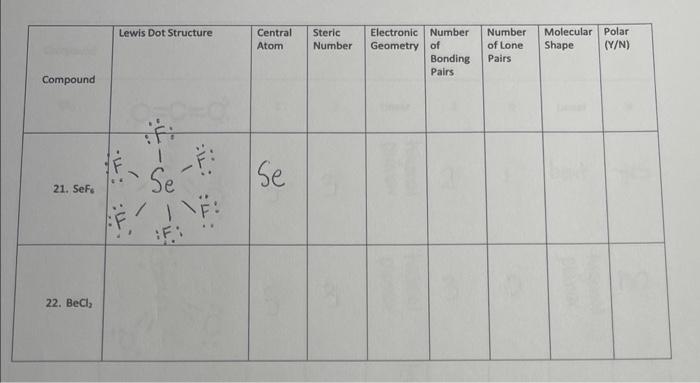

Compound 21. Sef 22. BeCl? Lewis Dot Structure TI: TI ( Se :T!: INF: Central Atom Se Steric Number Electronic Number Geometry of hal Bonding Pairs Number of Lone Pairs Molecular Polar (Y/N) Shape
1211 L postlab Calorimetry-in both cases ignore the calorimeter A 248-g sample of copper is dropped into 390 g of water at 22.6°C. The final temperature was measured to be 39.9°C. Calculate the initial temperature of the copper. Density of water 1.00g/mL. Specific heat of copper = .384 J/g °C When 50.0 mL of .10 M HCl and 50.0 mL of .10 M NaOH, both at 22 °C, are added to a calorimeter, the temperature of the mixture reaches 28.9 °C. Calculate the heat produced by this reaction. Density of water 1.00g/mL. Specific heat of water = 4.184 J/g °C