Home /
Expert Answers /
Chemistry /
complex-the-equilibrium-constant-for-the-reaction-is-called-is-the-formation-constant-k-mat-pa452
(Solved): complex, the equilibrium constant for the reaction is called is the formation constant, \( K_{\mat ...
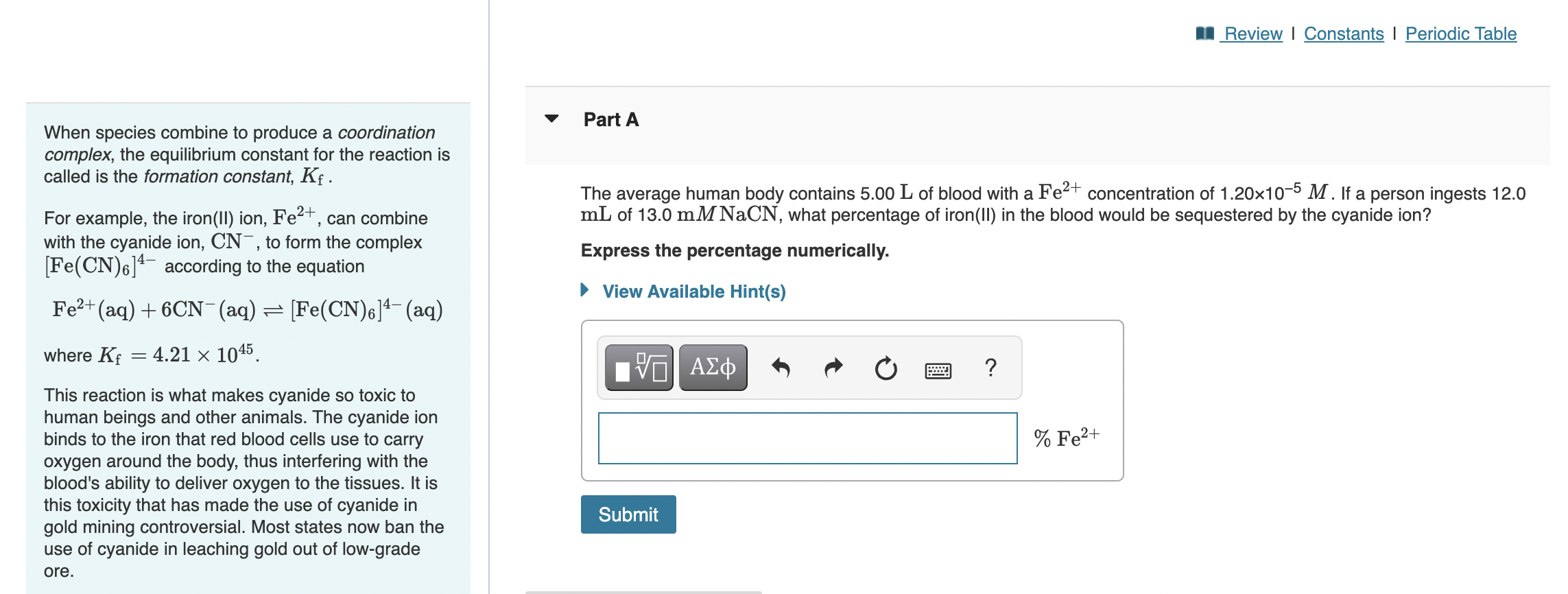
complex, the equilibrium constant for the reaction is called is the formation constant, \( K_{\mathrm{f}} \). For example, the iron(II) ion, \( \mathrm{Fe}^{2+} \), can combine with the cyanide ion, \( \mathrm{CN}^{-} \), to form the complex \( {\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-} \) according to the equation \( } \mathrm{Fe}^{2+}(\mathrm{aq})+6 \mathrm{CN}^{-}(\mathrm{aq}) \rightleftharpoons\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}(\mathrm{aq}) \) where \( K_{\mathrm{f}}=4.21 \times 10^{45} \). Express the percentage numerically. This reaction is what makes cyanide so toxic to human beings and other animals. The cyanide ion binds to the iron that red blood cells use to carry oxygen around the body, thus interfering with the blood's ability to deliver oxygen to the tissues. It is this toxicity that has made the use of cyanide in gold mining controversial. Most states now ban the use of cyanide in leaching gold out of low-grade ore.