Home /
Expert Answers /
Chemistry /
commercial-34-chlorine-34-clorox-cloralex-has-as-an-active-ingredient-sodium-hypochlorite-concentra-pa912
(Solved): Commercial "chlorine" (Clorox, Cloralex) has as an active ingredient sodium hypochlorite concentra ...
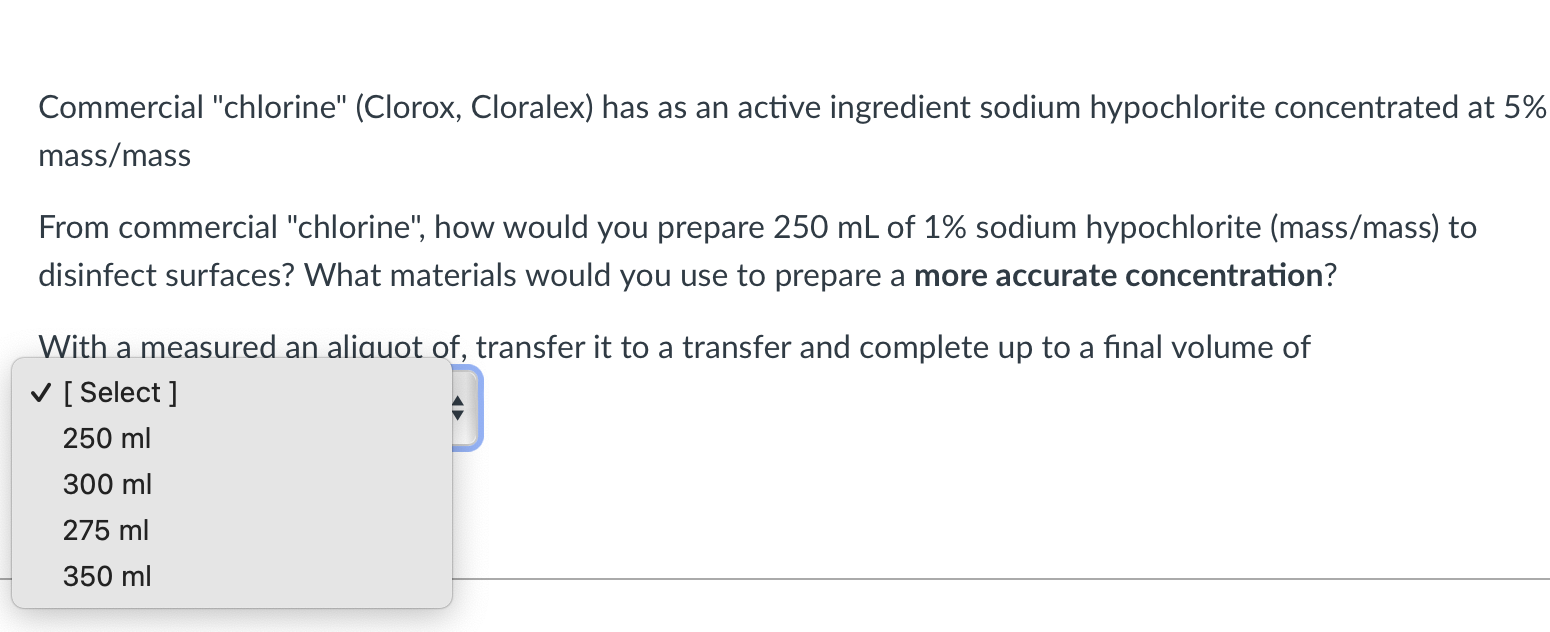
Commercial "chlorine" (Clorox, Cloralex) has as an active ingredient sodium hypochlorite concentrated at 5\% mass/mass From commercial "chlorine", how would you prepare \( 250 \mathrm{~mL} \) of \( 1 \% \) sodium hypochlorite (mass \( / \mathrm{mass} \) ) to disinfect surfaces? What materials would you use to prepare a more accurate concentration? With a measured an aliauot of, transfer it to a transfer and complete up to a final volume of
Expert Answer
Firstly, we calculate the volume of commercial chlorine that needs to be diluted, using the equa