Home /
Expert Answers /
Chemistry /
classify-the-following-reactions-as-synthesis-decomposition-single-displacement-or-double-displac-pa237
(Solved): Classify the following reactions as synthesis, decomposition, single-displacement, or double-displac ...
Classify the following reactions as synthesis, decomposition, single-displacement, or double-displacement reactions. Drag the items to their respective bins. ? View Available Hint(s) Mg + 2HCl MgCl2 + H? 2NaCl-2Na+ Cl? 2H?O-2H? + O? 8Fe+Sg 8FeS C6H12 + Br2 C6H12Br2 2Pb(NO3)2-2PbO + 4NO? + 02 Synthesis (combination) Decomposition MgCO3 + 2HCI? Single-displacement Pb(NO3)2 + 2NaCl ? 2NaNO3 + PbCl? Reset Help MgCl2 + H2CO3 Double-displacement
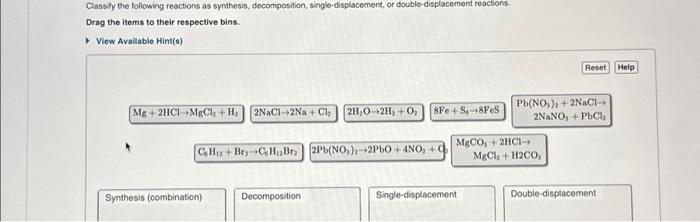
Classity the following reactions as synthesis, docomposition, single-displacement, or double-displacement reactions. Drag the liems to their respective bins. View Avallable Hint(s)