Home /
Expert Answers /
Chemical Engineering /
chlorine-dioxide-clo2-is-a-disinfectant-used-in-municipal-water-treatment-plants-it-dissolves-pa118
(Solved): Chlorine dioxide (ClO2) is a disinfectant used in municipal water treatment plants. It dissolves ...
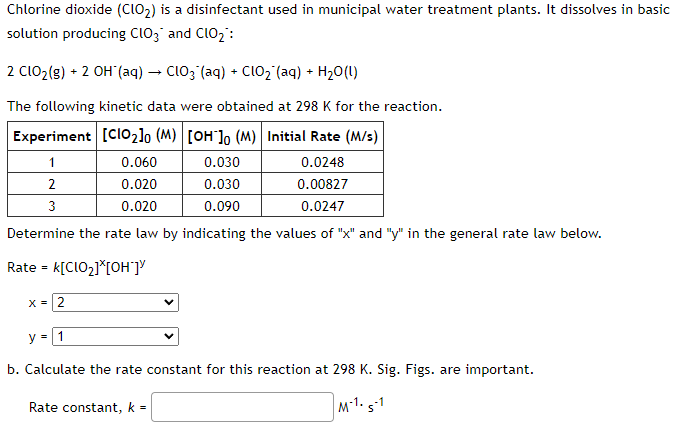
Chlorine dioxide is a disinfectant used in municipal water treatment plants. It dissolves in basic solution producing and : The following kinetic data were obtained at for the reaction. Determine the rate law by indicating the values of " " and " " in the general rate law below. Rate b. Calculate the rate constant for this reaction at . Sig. Figs. are important. Rate constant,
Expert Answer
To determine the rate constant (k) for the reaction, we can use the rate equation:Rate = k[CO2]^x[OH]^yUsing the given experimental data, we can choose any two experiments and set up a ratio to eliminate the concentration of CO2 or OH. Let's use experiments 1 and 2:(Rate1 / Rate2) = ([CO2]1^x[OH]1^y) / ([CO2]2^x[OH]2^y)Plugging in the values from the experiments: