Home /
Expert Answers /
Chemistry /
carbon-disulfide-mathrm-cs-2-burns-in-oxygen-complete-combustion-gives-the-balanced-r-pa876
(Solved): Carbon disulfide, \( \mathrm{CS}_{2} \), burns in oxygen. Complete combustion gives the balanced r ...
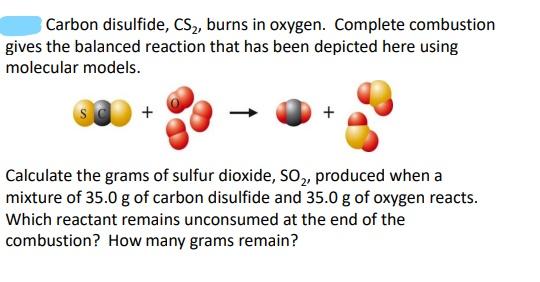
Carbon disulfide, \( \mathrm{CS}_{2} \), burns in oxygen. Complete combustion gives the balanced reaction that has been depicted here using molecular models. Calculate the grams of sulfur dioxide, \( \mathrm{SO}_{2} \), produced when a mixture of \( 35.0 \mathrm{~g} \) of carbon disulfide and \( 35.0 \mathrm{~g} \) of oxygen reacts. Which reactant remains unconsumed at the end of the combustion? How many grams remain?