Home /
Expert Answers /
Chemistry /
can-u-please-do-a-b-and-e-for-each-of-the-following-reactions-a-rewrite-the-reaction-on-a-separa-pa616
(Solved): can u please do a, b and e For each of the following reactions: a) Rewrite the reaction on a separa ...
can u please do a, b and e
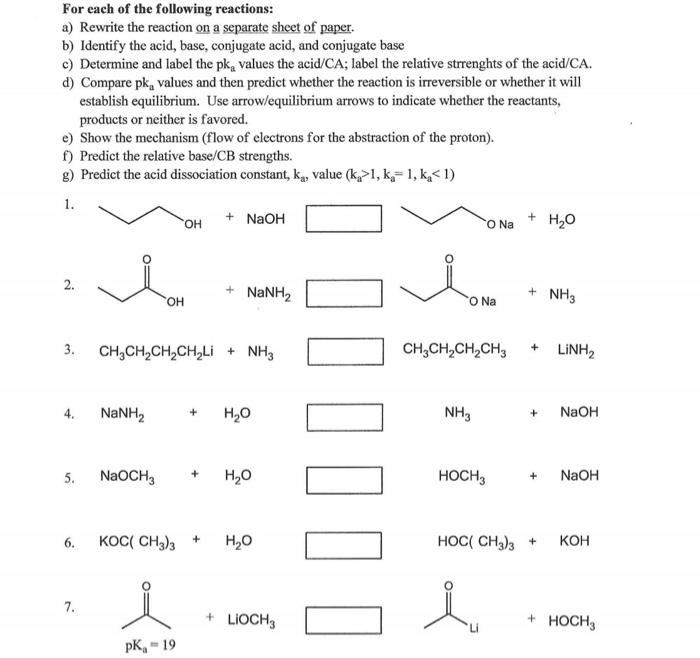
For each of the following reactions: a) Rewrite the reaction on a separate sheet of paper. b) Identify the acid, base, conjugate acid, and conjugate base c) Determine and label the \( \mathrm{pk}_{\mathrm{a}} \) values the acid/CA; label the relative strrenghts of the acid/CA. d) Compare \( \mathrm{pk}_{\mathrm{a}} \) values and then predict whether the reaction is irreversible or whether it will establish equilibrium. Use arrow/equilibrium arrows to indicate whether the reactants, products or neither is favored. e) Show the mechanism (flow of electrons for the abstraction of the proton). f) Predict the relative base/CB strengths. g) Predict the acid dissociation constant, \( \mathrm{k}_{\mathrm{a}} \), value \( \left(\mathrm{k}_{\mathrm{a}}>1, \mathrm{k}_{\mathrm{a}}=1, \mathrm{k}_{\mathrm{a}}<1\right) \) 1. \( \mathrm{OH}+\mathrm{NaOH} \) 2. 3. \( \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Li}+\mathrm{NH}_{3} \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CH}_{3}+\mathrm{LiNH}_{2} \) 4. \( \mathrm{NaNH}_{2}+\mathrm{H}_{2} \mathrm{O} \quad \mathrm{NH}_{3}+\mathrm{NaOH} \) 5. \( \mathrm{NaOCH}_{3}+\mathrm{H}_{2} \mathrm{O} \quad \mathrm{HOCH}_{3}+\mathrm{NaOH} \) 6. \( \mathrm{KOC}\left(\mathrm{CH}_{3}\right)_{3}+\mathrm{H}_{2} \mathrm{O} \quad \mathrm{HOC}\left(\mathrm{CH}_{3}\right)_{3}+\mathrm{KOH} \) 7.