Home /
Expert Answers /
Chemistry /
can-someone-help-with-part-a-b-and-c-dealing-with-orbitals-figures-given-below-atomic-orbitals-pa152
(Solved): Can someone help with Part A, B and C dealing with orbitals? Figures given below: Atomic orbitals ...
Can someone help with Part A, B and C dealing with orbitals? Figures given below:
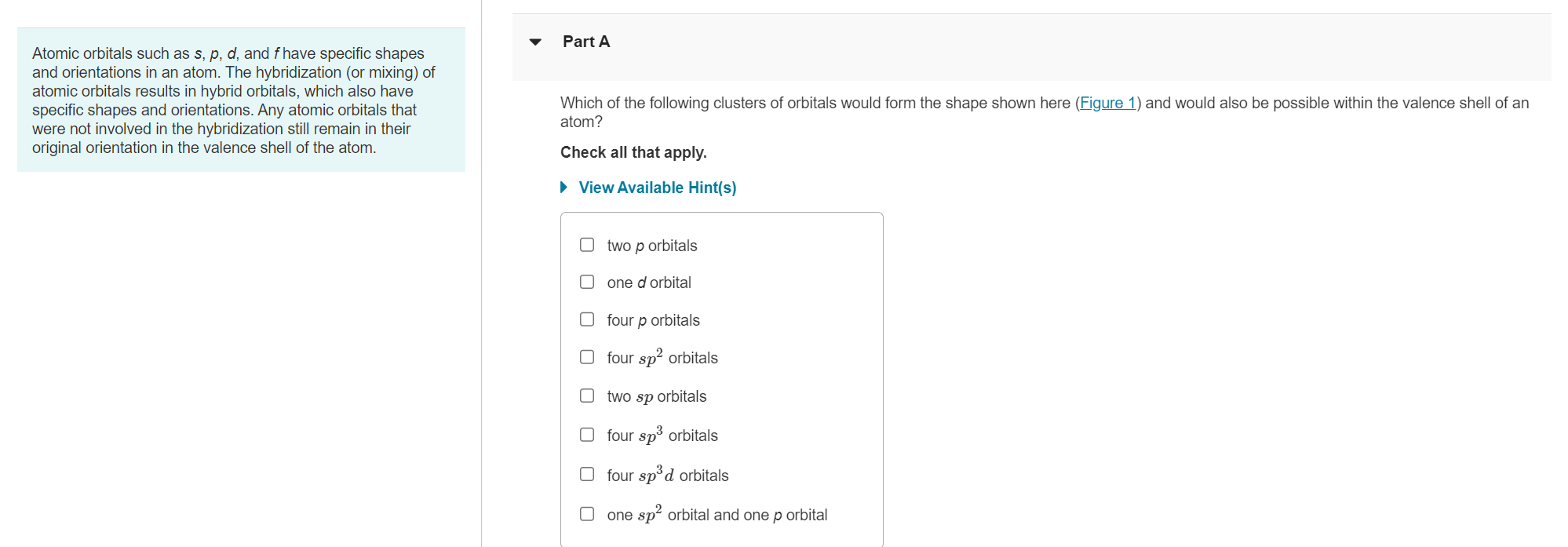
Atomic orbitals such as \( s, p, d \), and \( f \) have specific shapes Part A and orientations in an atom. The hybridization (or mixing) of atomic orbitals results in hybrid orbitals, which also have specific shapes and orientations. Any atomic orbitals that Which of the following clusters of orbitals would form the shape shown here (Figure 1) and would also be possible within the valence shell of an were not involved in the hybridization still remain in their atom? original orientation in the valence shell of the atom. Check all that apply. View Available Hint(s) two \( p \) orbitals one \( d \) orbital four \( p \) orbitals four \( s p^{2} \) orbitals two \( s p \) orbitals four \( s p^{3} \) orbitals four \( s p^{3} d \) orbitals one \( s p^{2} \) orbital and one \( p \) orbital
Figure 1 of 3
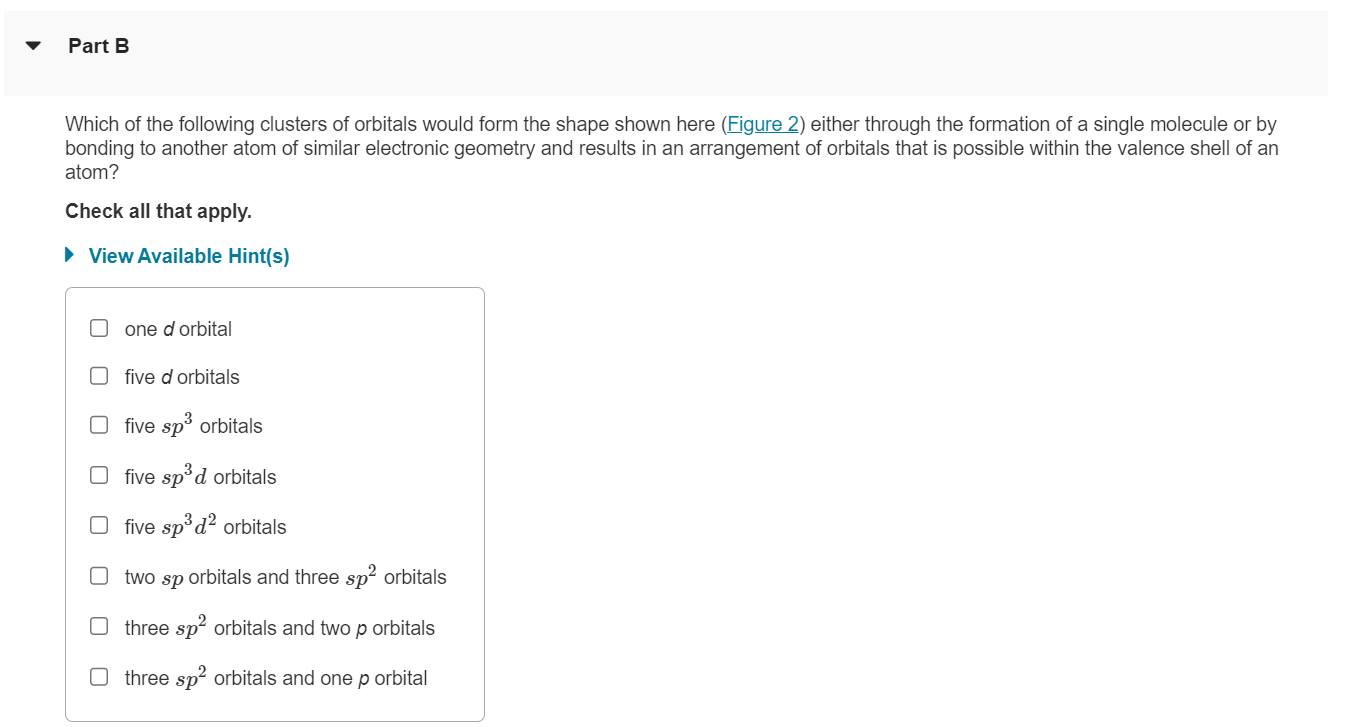
Which of the following clusters of orbitals would form the shape shown here (Figure 2 ) either through the formation of a single molecule or by bonding to another atom of similar electronic geometry and results in an arrangement of orbitals that is possible within the valence shell of an atom? Check all that apply. View Available Hint(s) one \( d \) orbital five \( d \) orbitals five \( s p^{3} \) orbitals five \( s p^{3} d \) orbitals five \( s p^{3} d^{2} \) orbitals two \( s p \) orbitals and three \( s p^{2} \) orbitals three \( s p^{2} \) orbitals and two \( p \) orbitals three \( s p^{2} \) orbitals and one \( p \) orbital
Figure < 2 of 3
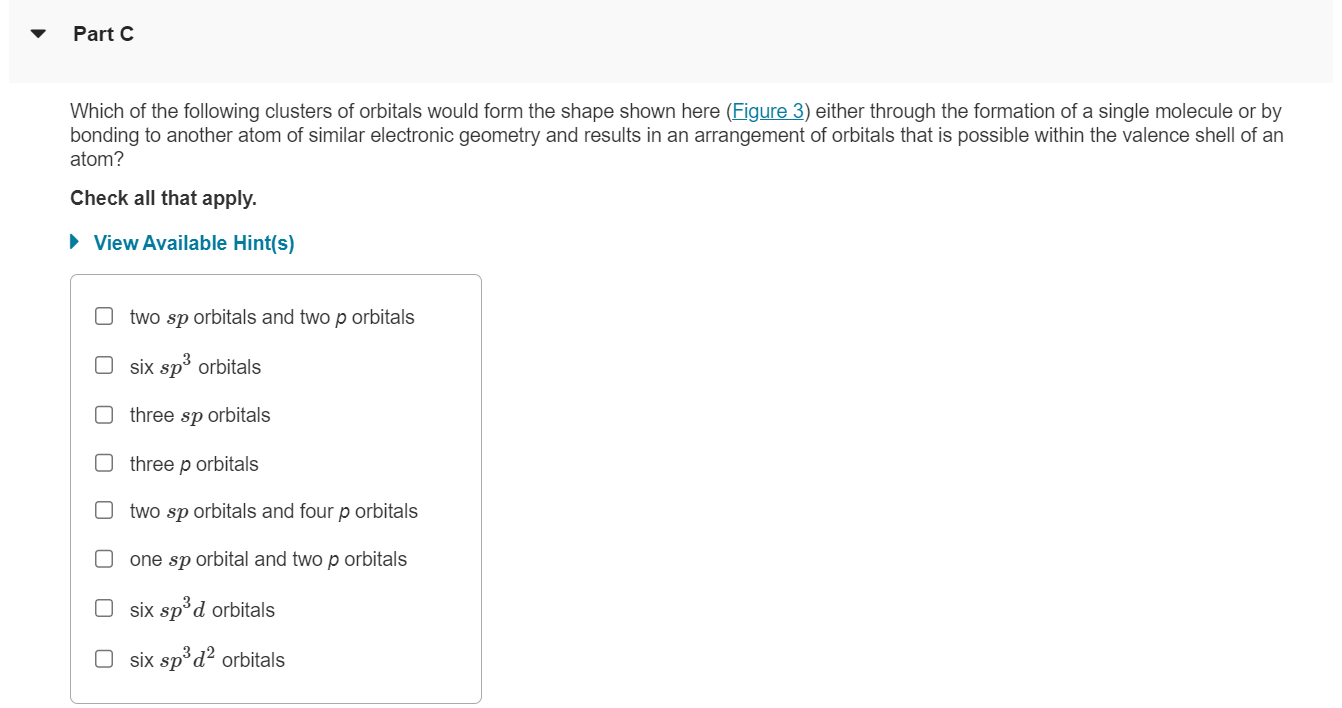
Which of the following clusters of orbitals would form the shape shown here (Figure 3 ) either through the formation of a single molecule or by bonding to another atom of similar electronic geometry and results in an arrangement of orbitals that is possible within the valence shell of an atom? Check all that apply. View Available Hint(s) two \( s p \) orbitals and two \( p \) orbitals six \( s p^{3} \) orbitals three \( s p \) orbitals three \( p \) orbitals two \( s p \) orbitals and four \( p \) orbitals one \( s p \) orbital and two \( p \) orbitals six \( s p^{3} d \) orbitals \( \operatorname{six} s p^{3} d^{2} \) orbitals
3 of 3