Home /
Expert Answers /
Chemistry /
can-someone-help-me-with-this-answer-cpost-lecture-homework-chapter-10-pm-calculating-the-pa631
(Solved): can someone help me with this answer? CPost Lecture Homework Chapter 10 \( \pm \) Calculating the \( ...
can someone help me with this answer?
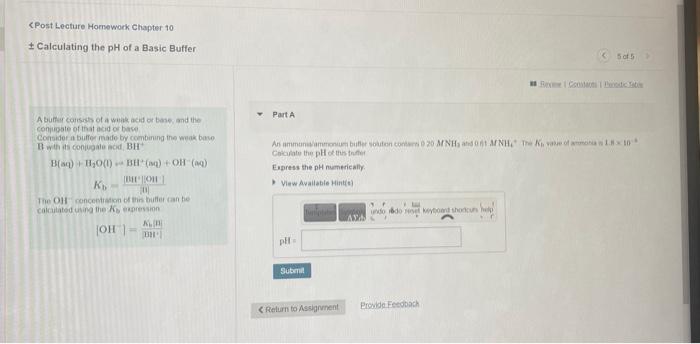

CPost Lecture Homework Chapter 10 \( \pm \) Calculating the \( \mathrm{pH} \) of a Basic Buffer A bufter corrests of a winak acid or base and the conimipate of that acid or bace. Consider a buiffer made by combirung ithe weak bowe. \( \mathrm{B}_{\text {with is }} \) is conivgale acid \( \mathrm{BH}^{+} \) \( \mathrm{H}(\mathrm{aq})+\mathrm{H}_{2} \mathrm{O}(\mathrm{I})+\mathrm{BH}^{2}(\mathrm{amg})+\mathrm{OH}^{-}(\mathrm{aqg}) \) Cocalate the \( \mathrm{pH} \) octhe tnfer Eupress the \( \mathrm{nH} \) numerically. \( K_{b} \frac{[44 !|69 !|}{\text { 11] }} \) 1 Tie OH roncentiateri of thin butler tan fee colnutated uning the \( \mathcal{K}_{\mathrm{b}} \) espressin \[ \left\{\mathrm{OH} \mid=\frac{\kappa_{n} \mathrm{~B}}{\mathrm{BH}}\right. \]