Home /
Expert Answers /
Chemistry /
can-someone-explain-these-concepts-to-me1-2-nbsp-3-which-complex-has-the-weakest-carbon-oxygen-bo-pa616
(Solved): can someone explain these concepts to me1)2) 3) Which complex has the weakest carbon-oxygen bo ...
can someone explain these concepts to me
1)
![Which complex has the weakest carbon-oxygen bood due to backbonding?
(A) \( \left[\mathrm{Ti}(\mathrm{CO})_{6}\right]^{2-} \)](https://media.cheggcdn.com/study/b5c/b5c32a3c-2830-4713-8f79-3f144bf35167/image)
2)
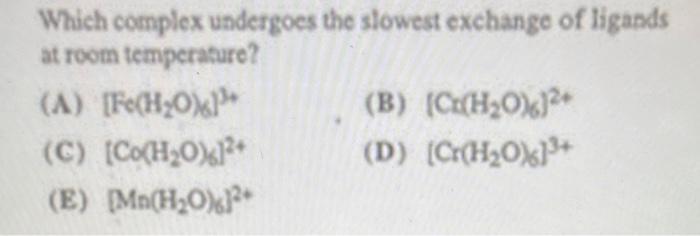
3)


Which complex has the weakest carbon-oxygen bood due to backbonding? (A) \( \left[\mathrm{Ti}(\mathrm{CO})_{6}\right]^{2-} \) (B) \( \mathrm{Cr}(\mathrm{CO}) \mathrm{s} \) (C) \( \left[\mathrm{Mn}(\mathrm{CO})_{6}\right]^{*} \) (D) \( \left[\mathrm{Fe}(\mathrm{CO})_{\mathrm{A}}\right]^{\mathrm{P}} \)
Which complex undergoes the slowest exchange of ligands at room temperature? (A) \( \left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right){ }^{3}\right]^{3+} \) (B) \( \left(\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right)^{2 t} \) (C) \( \left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+} \) (D) \( \left[\mathrm{Cr}\left(\mathrm{H}_{2} \mathrm{O}_{6}\right]^{3+}\right. \) (E) \( \left[\mathrm{Mn}\left(\mathrm{H}_{2} \mathrm{O}\right)\right]^{2+} \)
Which species has the greatest tendency to undergo oxidative addition of \( \mathrm{HCl} \) ? ( \( \mathrm{Me} \) is \( \mathrm{CH}_{3} \).) (A) \( \left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right) 6\right]^{3+} \) (B) \( \left[\mathrm{CoCl}_{2}\left(\mathrm{NH}_{3}\right)_{4}\right]^{+} \) (C) \( \left[\mathrm{HCl}(\mathrm{CO})\left(\mathrm{PM}_{\mathrm{M}}\right)_{2}\right] \) (D) \( \left[\mathrm{NiBr}_{4}\right]^{2-} \) (E) \( \left[\mathrm{AgCl}_{2}\right]^{-} \)
can someone explain these concepts to me 1) Which Complex has the weakest carbon-oxygen bond due to backbonding? Which complex has the weakest carbon-oxygen bond due to backbonding? (B) \( \mathrm{CrCO}_{2} \) (B) \( C \) rico (C) \( \left[\mathrm{Mn}(\mathrm{CO})_{\mathrm{d}} \mathrm{d}^{*}(\mathrm{C})\left(\mathrm{Mn}(\mathrm{CO})_{\mathrm{a}}\right]^{+}\right. \) (D) \( [\mathrm{Fec}(\mathrm{CO})]^{\text {i* }}(\mathrm{D})\left[\mathrm{fe}(\mathrm{CO})_{\mathrm{a}}\right]^{2} \) 2) Which Complex undergoes the slowest exchange of ligands at room temperature? Which complex usdergoes the sloweat excbange of ligands at room temperature? (E) \( \left[\mathrm{Mn}\left(\mathrm{H}_{2} \mathrm{O}\right)\right]^{2+}(\mathrm{E})\left[\mathrm{Mn}\left(\mathrm{H} 2 \mathrm{O}_{6}\right]^{2}\right. \) 3) Which species has the greatest tendency to undergo oxidative addition of \( \mathrm{HCl} \) ? ( \( \mathrm{Me} \) is \( \mathrm{CH}_{3} \) )? Which species has the greatest tendency to undergo oxidative addition of \( \mathrm{HCl} \) ? \( \left(\mathrm{Me}\right. \) is \( \left.\mathrm{CH}_{3}\right) \) (A) \( \left\{\left(\mathrm{Cr}\left(\mathrm{NH}_{3}\right) 6\right\}^{3+}(\mathrm{A})\left(\mathrm{Gr} \mathrm{NH}_{3}\right)_{6}{ }^{3}\right. \) (B) \( \left[\mathrm{CoCl}_{2}\left(\mathrm{NH}_{3}\right)_{4}\right]^{*} \) (B) \( \left[\mathrm{COCl}_{2}\left(\mathrm{NH}_{3}\right) \mathrm{d}\right] \) (D) \( \left[\mathrm{NiBr}_{a}\right]^{2-} \) (D) NiBride \( ^{2} \) (E) \( \left[\mathrm{AgCl}_{2}\right]^{-} \)
Expert Answer
Option (D) In case of [Fe(CO)5] the electronic configuratio