Home /
Expert Answers /
Chemistry /
calculate-the-volume-in-milliliters-of-a-1-81-mathrm-mol-mathrm-l-nickel-ii-chloride-pa322
(Solved): Calculate the volume in milliliters of a \( 1.81 \mathrm{~mol} / \mathrm{L} \) nickel(II) chloride ...
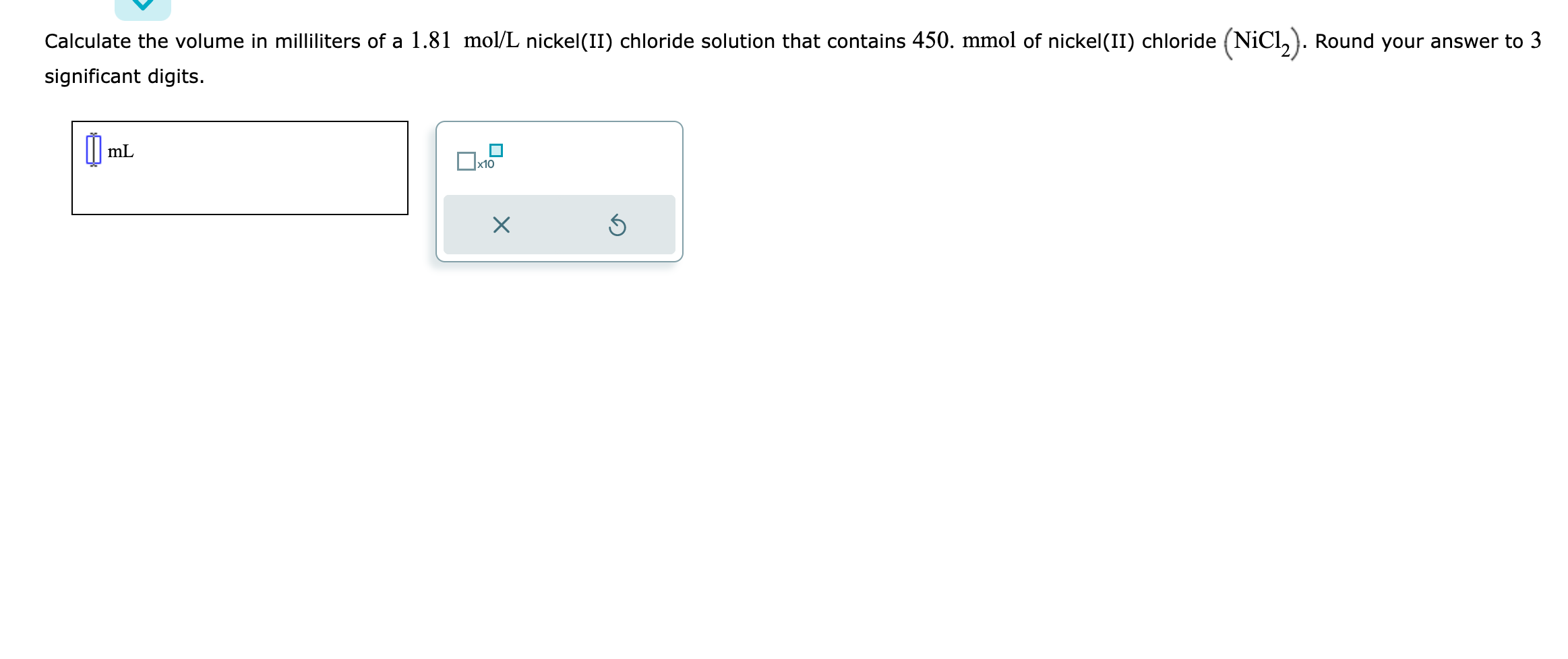
Calculate the volume in milliliters of a \( 1.81 \mathrm{~mol} / \mathrm{L} \) nickel(II) chloride solution that contains \( 450 . \mathrm{mmol} \) of nickel(II) chloride ( \( \mathrm{NiCl} 2 \) ). Round your answer to 3 significant digits.