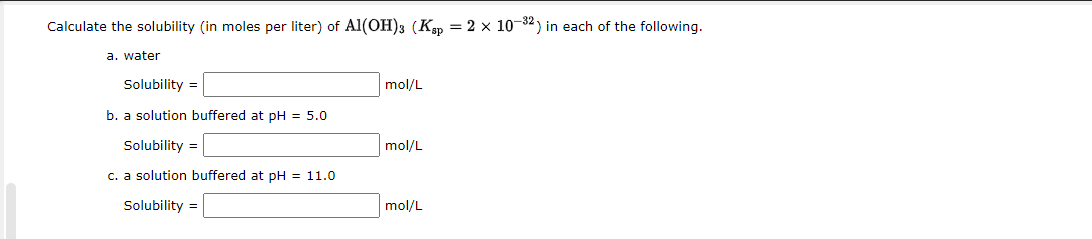Home /
Expert Answers /
Chemistry /
calculate-the-solubility-in-moles-per-liter-of-mathrm-al-mathrm-oh-3-left-k-mathrm-s-pa907
(Solved): Calculate the solubility (in moles per liter) of \( \mathrm{Al}(\mathrm{OH})_{3}\left(K_{\mathrm{s ...
Calculate the solubility (in moles per liter) of \( \mathrm{Al}(\mathrm{OH})_{3}\left(K_{\mathrm{sp}}=2 \times 10^{-32}\right) \) in each of the following. a. water Solubility \( = \) \( \mathrm{mol} / \mathrm{L} \) b. a solution buffered at \( \mathrm{pH}=5.0 \) Solubility \( = \) \( \mathrm{mol} / \mathrm{L} \) c. a solution buffered at \( \mathrm{pH}=11.0 \) Solubility \( = \) \( \mathrm{mol} / \mathrm{L} \)
Expert Answer
A) Al(OH)3 is insoluble in water so the solubility will be zero. B) Al(OH)3 Al3+ + 3 OH- S 3x Ksp