Home /
Expert Answers /
Chemical Engineering /
calculate-the-pren-values-by-using-the-nace-mr-0175-iso-15156-pren-equation-nbsp-pren-cr-n-pa301
(Solved): - Calculate the PREN values by using the NACE (MR 0175/ISO 15156) PREN equation: PREN = %Cr&n ...
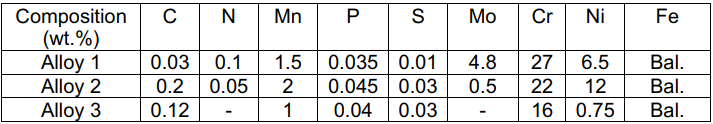
- Calculate the PREN values by using the NACE (MR 0175/ISO 15156) PREN equation: PREN = %Cr + 3.3(%Mo + 0.5%W) + 16%N
- If the temperature is increased, what would happen to the passive oxide film of these stainless steels?
\begin{tabular}{|c|c|c|c|c|c|c|c|c|c|} \hline Composition (wt.\%) & \( \mathrm{C} \) & \( \mathrm{N} \) & \( \mathrm{Mn} \) & \( \mathrm{P} \) & \( \mathrm{S} \) & \( \mathrm{Mo} \) & \( \mathrm{Cr} \) & \( \mathrm{Ni} \) & \( \mathrm{Fe} \) \\ \hline Alloy 1 & \( 0.03 \) & \( 0.1 \) & \( 1.5 \) & \( 0.035 \) & \( 0.01 \) & \( 4.8 \) & 27 & \( 6.5 \) & Bal. \\ \hline Alloy 2 & \( 0.2 \) & \( 0.05 \) & 2 & \( 0.045 \) & \( 0.03 \) & \( 0.5 \) & 22 & 12 & Bal. \\ \hline Alloy 3 & \( 0.12 \) & \( - \) & 1 & \( 0.04 \) & \( 0.03 \) & \( - \) & 16 & \( 0.75 \) & Bal. \\ \hline \end{tabular}