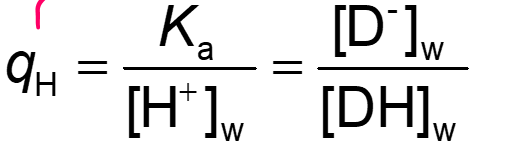Home /
Expert Answers /
Chemistry /
calculate-the-pka-value-of-an-acidic-drug-if-at-ph-7-4-1-the-apparent-partition-coefficient-is-pa302
(Solved): Calculate the pKa value of an acidic drug if, at pH = 7.4: (1) the apparent partition coefficient is ...
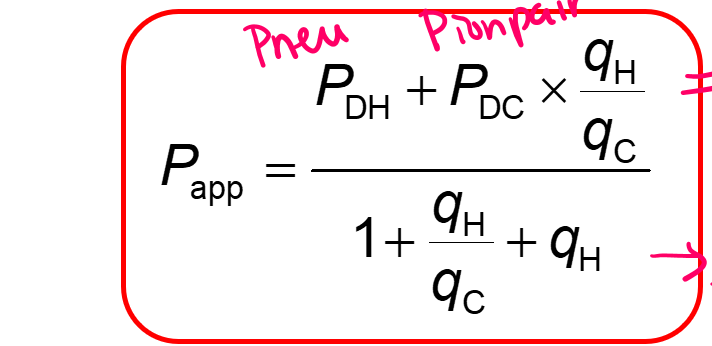
Calculate the pKa value of an acidic drug if, at pH = 7.4: (1) the apparent partition coefficient is 3.17 x 103; (2) the concentration of the nonionized molecules is twice the concentration of ion pairs, both in the aqueous phase. The partition coefficients of the nonionized drug and of the ion pair are 7.24 x 103 and 3.01 x 103, respectively.
Use formulas: