Home /
Expert Answers /
Chemistry /
calculate-the-percent-yield-for-the-reaction-shown-below-by-constructing-a-bca-table-determining-pa820
(Solved): Calculate the percent yield for the reaction shown below by constructing a BCA table, determining ...
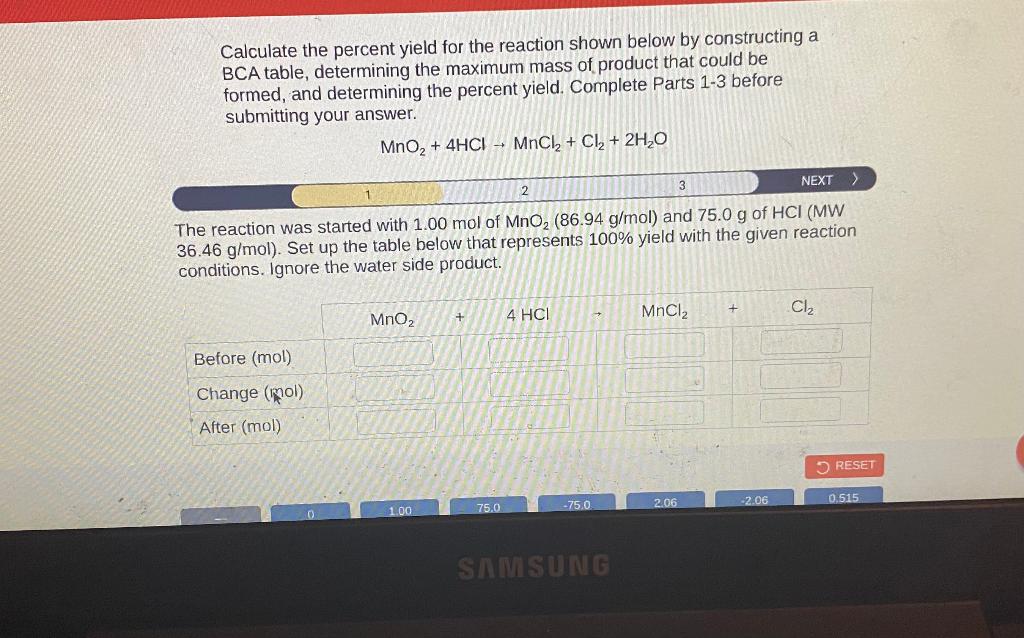
Calculate the percent yield for the reaction shown below by constructing a BCA table, determining the maximum mass of product that could be formed, and determining the percent yield. Complete Parts 1-3 before submitting your answer. \[ \mathrm{MnO}_{2}+4 \mathrm{HCl} \rightarrow \mathrm{MnCl}_{2}+\mathrm{Cl}_{2}+2 \mathrm{H}_{2} \mathrm{O} \] he reaction was started with \( 1.00 \mathrm{~mol} \) of \( \mathrm{MnO}_{2}(86.94 \mathrm{~g} / \mathrm{mol}) \) and \( 75.0 \mathrm{~g} \) of HCl (MW \( 36.46 \mathrm{~g} / \mathrm{mol}) \). Set up the table below that represents \( 100 \% \) yield with the given reaction conditions. Ignore the water side product.