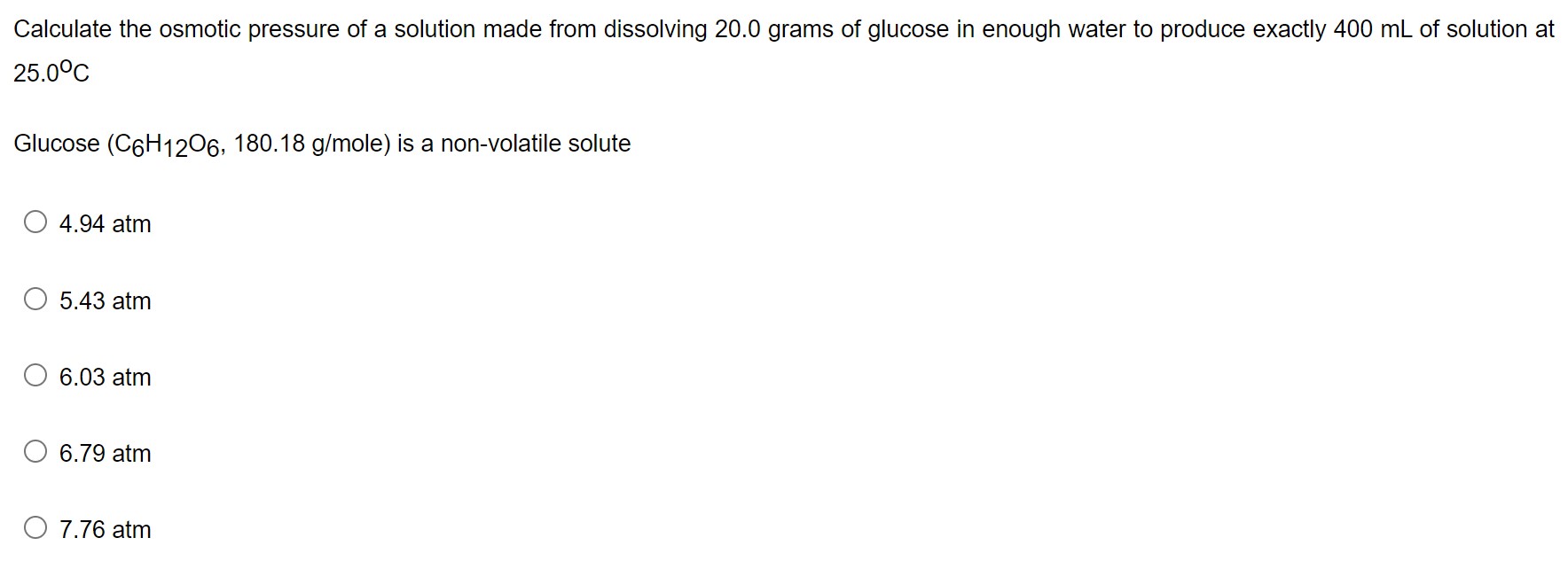Home /
Expert Answers /
Chemistry /
calculate-the-osmotic-pressure-of-a-solution-made-from-dissolving-20-0-grams-of-glucose-in-enough-w-pa368
Expert Answer
Given data: Mass of glucose = 20.0 g Volume of the solution = 400 mL = 0.4 L Temperature = 12.0°C = ...