Home /
Expert Answers /
Chemistry /
calculate-the-number-of-moles-of-carbon-atoms-present-in-each-of-the-following-samples-a-1-26-pa920
(Solved): Calculate the number of moles of carbon atoms present in each of the following samples. (a) \( 1.26 ...
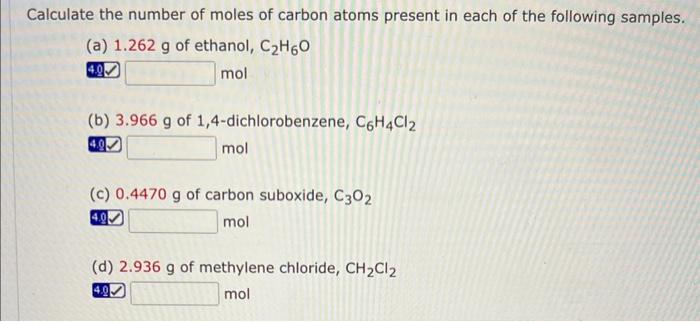
Calculate the number of moles of carbon atoms present in each of the following samples. (a) \( 1.262 \mathrm{~g} \) of ethanol, \( \mathrm{C}_{2} \mathrm{H}_{6} \mathrm{O} \) \( \mathrm{mol} \) (b) \( 3.966 \mathrm{~g} \) of 1,4-dichlorobenzene, \( \mathrm{C}_{6} \mathrm{H}_{4} \mathrm{Cl}_{2} \) mol (c) \( 0.4470 \mathrm{~g} \) of carbon suboxide, \( \mathrm{C}_{3} \mathrm{O}_{2} \) \( \mathrm{mol} \) (d) \( 2.936 \mathrm{~g} \) of methylene chloride, \( \mathrm{CH}_{2} \mathrm{Cl}_{2} \) \( \mathrm{mol} \)
Expert Answer
a. no of moles of C2H6O = W/G.M.Wt = 1.262/46 = 0.0274moles 1 mole of C2H6O contains 2 moles of C 0.0274 moles of C2H6O contains = 2