Home /
Expert Answers /
Chemistry /
calculate-the-number-of-moles-of-2-methyl-2-butanol-and-hydrochloric-acid-concentrated-hcl-is-12-m-pa903
(Solved): Calculate the number of moles of 2-methyl-2-butanol and hydrochloric acid (concentrated HCl is 12 M) ...
Calculate the number of moles of 2-methyl-2-butanol and hydrochloric
acid (concentrated HCl is 12 M). Based on the balanced equation, determine the limiting reagent and the
theoretical yield of 2-chloro-2-methylbutane and record it in your notebook as part of your prelab.
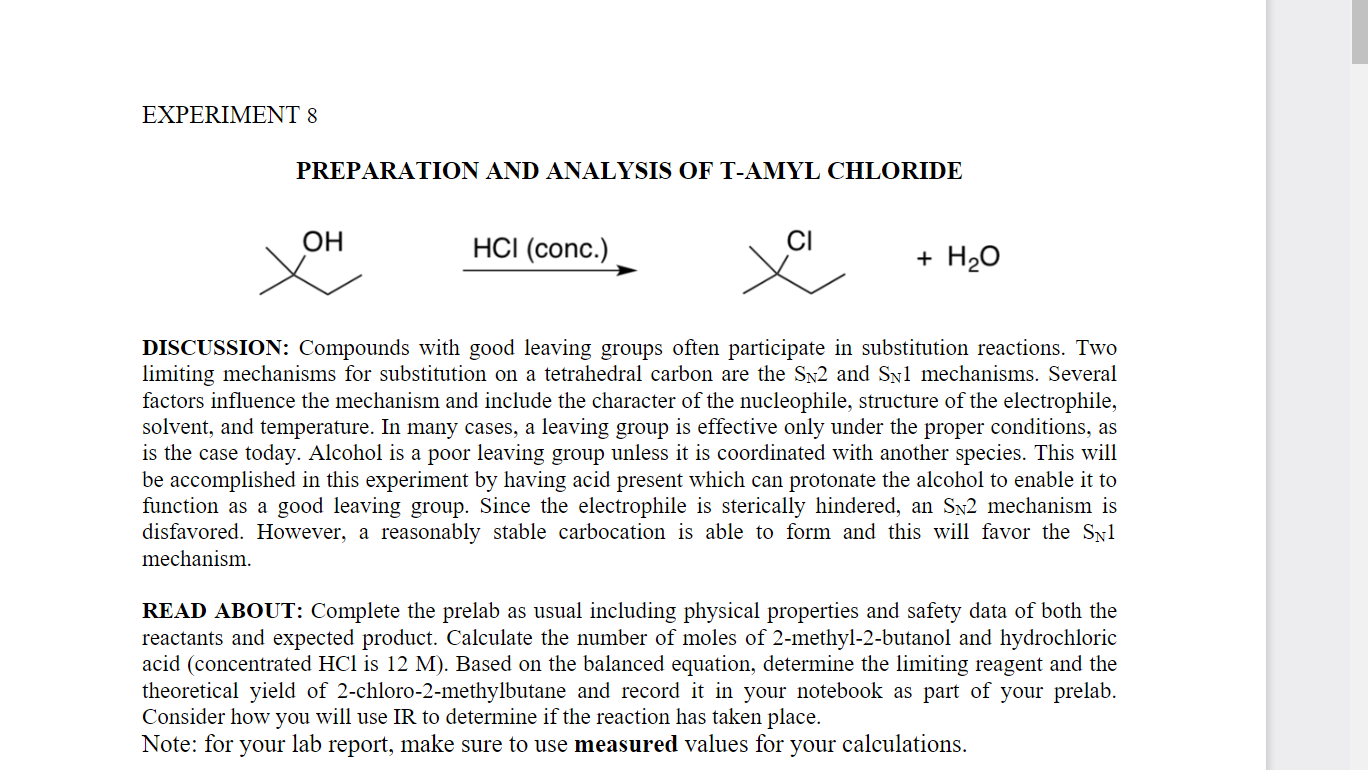
PREPARATION AND ANALYSIS OF T-AMYL CHLORIDE DISCUSSION: Compounds with good leaving groups often participate in substitution reactions. Two limiting mechanisms for substitution on a tetrahedral carbon are the \( S_{N} 2 \) and \( S_{N} 1 \) mechanisms. Several factors influence the mechanism and include the character of the nucleophile, structure of the electrophile, solvent, and temperature. In many cases, a leaving group is effective only under the proper conditions, as is the case today. Alcohol is a poor leaving group unless it is coordinated with another species. This will be accomplished in this experiment by having acid present which can protonate the alcohol to enable it to function as a good leaving group. Since the electrophile is sterically hindered, an \( \mathrm{S}_{\mathrm{N}} 2 \) mechanism is disfavored. However, a reasonably stable carbocation is able to form and this will favor the \( \mathrm{S}_{\mathrm{N}} 1 \) mechanism. READ ABOUT: Complete the prelab as usual including physical properties and safety data of both the reactants and expected product. Calculate the number of moles of 2-methyl-2-butanol and hydrochloric acid (concentrated \( \mathrm{HCl} \) is \( 12 \mathrm{M} \) ). Based on the balanced equation, determine the limiting reagent and the theoretical yield of 2-chloro-2-methylbutane and record it in your notebook as part of your prelab. Consider how you will use IR to determine if the reaction has taken place. Note: for your lab report, make sure to use measured values for your calculations.
Expert Answer
Density = 0.805 g/mL Volume = 10 mL Molar mass of 2-methyl-2-butanol = 88.15 g/mol Molar mass of 2-chloro-2-methylbutane = 106 g/mol Step-1 Limiting reactant = 2-m