Home /
Expert Answers /
Chemistry /
calculate-the-net-energy-change-in-kilojoules-per-mole-that-takes-place-on-formation-of-mgcl2-s-pa264
(Solved): Calculate the net energy change in kilojoules per mole that takes place on formation of MgCl2(s) ...
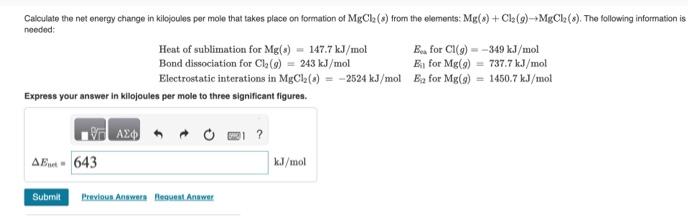
Calculate the net energy change in kilojoules per mole that takes place on formation of from the elements: . The following information is noedod: Heat of sublimation for for Bond dissociation for for Electrostatic interations in for Express your answer in kilojoules per mole to three significant figures.