Home /
Expert Answers /
Chemistry /
calculate-the-molar-solubility-of-silver-thiocyanate-agscn-in-pure-water-k-1-0-x-10-12-solubil-pa625
(Solved): Calculate the molar solubility of silver thiocyanate, AgSCN, in pure water (K-1.0 x 10-12). Solubil ...
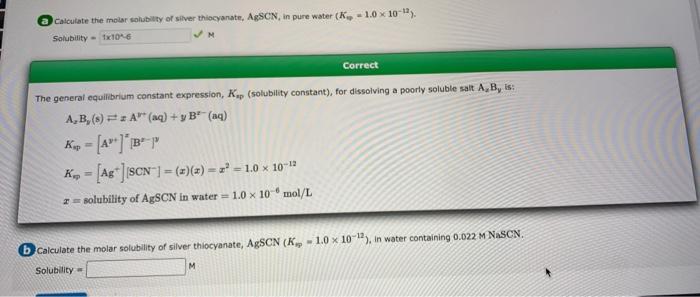
Calculate the molar solubility of silver thiocyanate, AgSCN, in pure water (K-1.0 x 10-12). Solubility 1x10^6 ?M The general equilibrium constant expression, Ksp (solubility constant), for dissolving a poorly soluble salt A, B, is: A,B,(s) A (aq) + y B² (aq) Kup = K=[Ag] [SCN] = (2) (2) = z² = 1.0 × 10-12 2= solubility of AgSCN in water = 1.0 x 10 mol/L Correct b calculate the molar solubility of silver thiocyanate, AgSCN (K 1.0 x 10-12), in water containing 0.022 M NaSCN. Solubility M
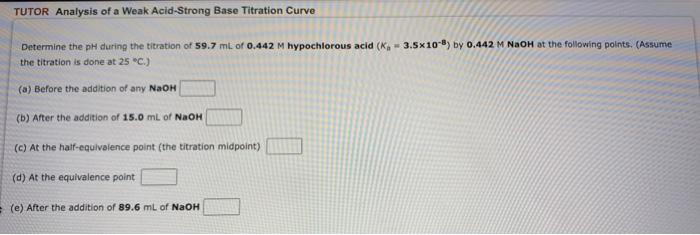
TUTOR Analysis of a Weak Acid-Strong Base Titration Curve W Determine the pH during the titration of 59.7 ml of 0.442 M hypochlorous acid (K, 3.5x108) by 0.442 M NaOH at the following points. (Assume the titration is done at 25 °C.) (a) Before the addition of any NaOH (b) After the addition of 15.0 mL of NaOH (c) At the half-equivalence point (the titration midpoint) (d) At the equivalence point (e) After the addition of 89.6 mL of NaOH
Expert Answer
b) Calculate the molar solubility of silver thiocyanate (Ksp=1.0E-12) in water containing 0.022 M NaSCN In this example, there ion SCN- is already present in the solution, form the NaSCN dissolved previously. Since NaSCN is a strong electrolyte, all