Home /
Expert Answers /
Statistics and Probability /
calculate-the-maximum-wavelength-of-light-capable-of-removing-an-electron-for-a-hydrogen-atom-from-pa175
(Solved): Calculate the maximum wavelength of light capable of removing an electron for a hydrogen atom from ...
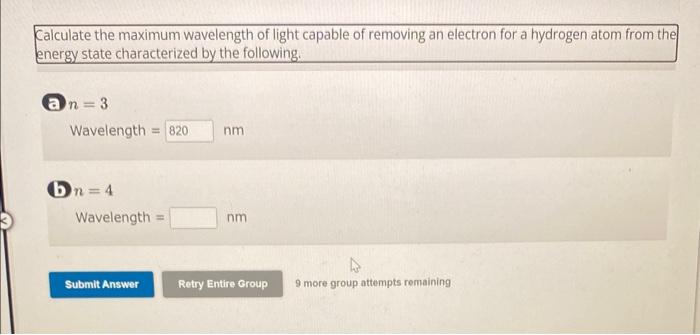
Calculate the maximum wavelength of light capable of removing an electron for a hydrogen atom from the energy state characterized by the following. ( Wavelength (b) Wavelength 9 more group attempts remaining
Expert Answer
The maximum wavelength of light that can remove an electron from a hydrogen atom in the