Home /
Expert Answers /
Chemistry /
calculate-the-maximum-grams-of-product-for-the-reaction-described-below-by-constructing-a-mathr-pa409
(Solved): Calculate the maximum grams of product for the reaction described below by constructing a \( \mathr ...
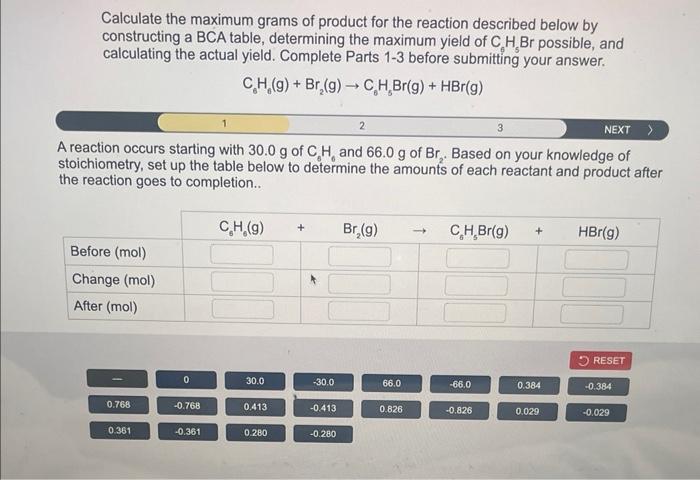
Calculate the maximum grams of product for the reaction described below by constructing a \( \mathrm{BCA} \) table, determining the maximum yield of \( \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{Br} \) possible, and calculating the actual yield. Complete Parts 1-3 before submitting your answer. \[ \mathrm{C}_{6} \mathrm{H}_{6}(\mathrm{~g})+\mathrm{Br}_{2}(\mathrm{~g}) \rightarrow \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{Br}(\mathrm{g})+\mathrm{HBr}(\mathrm{g}) \] A reaction occurs starting with \( 30.0 \mathrm{~g} \) of \( \mathrm{C}_{6} \mathrm{H}_{6} \) and \( 66.0 \mathrm{~g}^{\text {of }} \mathrm{Br}_{2} \). Based on your knowledge of stoichiometry, set up the table below to determine the amounts of each reactant and product after the reaction goes to completion..
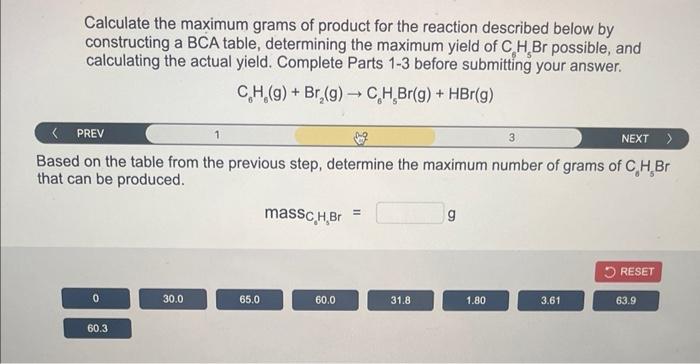
Calculate the maximum grams of product for the reaction described below by constructing a BCA table, determining the maximum yield of \( \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{Br} \) possible, and calculating the actual yield. Complete Parts 1-3 before submitting your answer. \[ \mathrm{C}_{6} \mathrm{H}_{6}(\mathrm{~g})+\mathrm{Br}_{2}(\mathrm{~g}) \rightarrow \mathrm{C}_{6} \mathrm{H}_{\mathrm{g}} \mathrm{Br}(\mathrm{g})+\mathrm{HBr}(\mathrm{g}) \] ? PREV ( ased on the table from the previous step, determine the maximum number of grams of \( \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{Br} \) nat can be produced. \[ \operatorname{mass}_{\mathrm{C}_{\mathrm{a}} \mathrm{H}_{\mathrm{a}} \mathrm{Br}}=\mathrm{g} \]
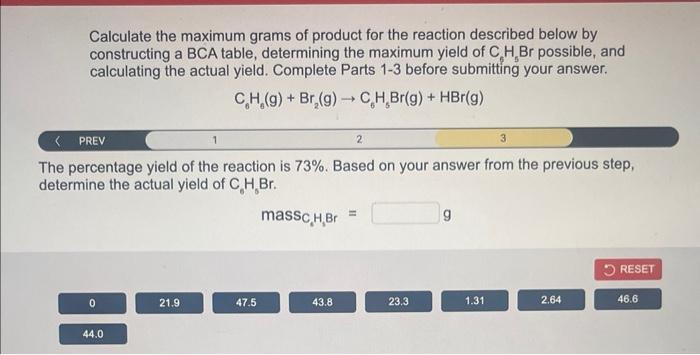
Calculate the maximum grams of product for the reaction described below by constructing a \( \mathrm{BCA} \) table, determining the maximum yield of \( \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{Br} \) possible, and calculating the actual yield. Complete Parts 1-3 before submitting your answer. \[ \mathrm{C}_{6} \mathrm{H}_{6}(\mathrm{~g})+\mathrm{Br}_{2}(\mathrm{~g}) \rightarrow \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{Br}(\mathrm{g})+\mathrm{HBr}(\mathrm{g}) \] The percentage yield of the reaction is \( 73 \% \). Based on your answer from the previous step, determine the actual yield of \( \mathrm{C}_{6} \mathrm{H}_{0} \mathrm{Br} \).