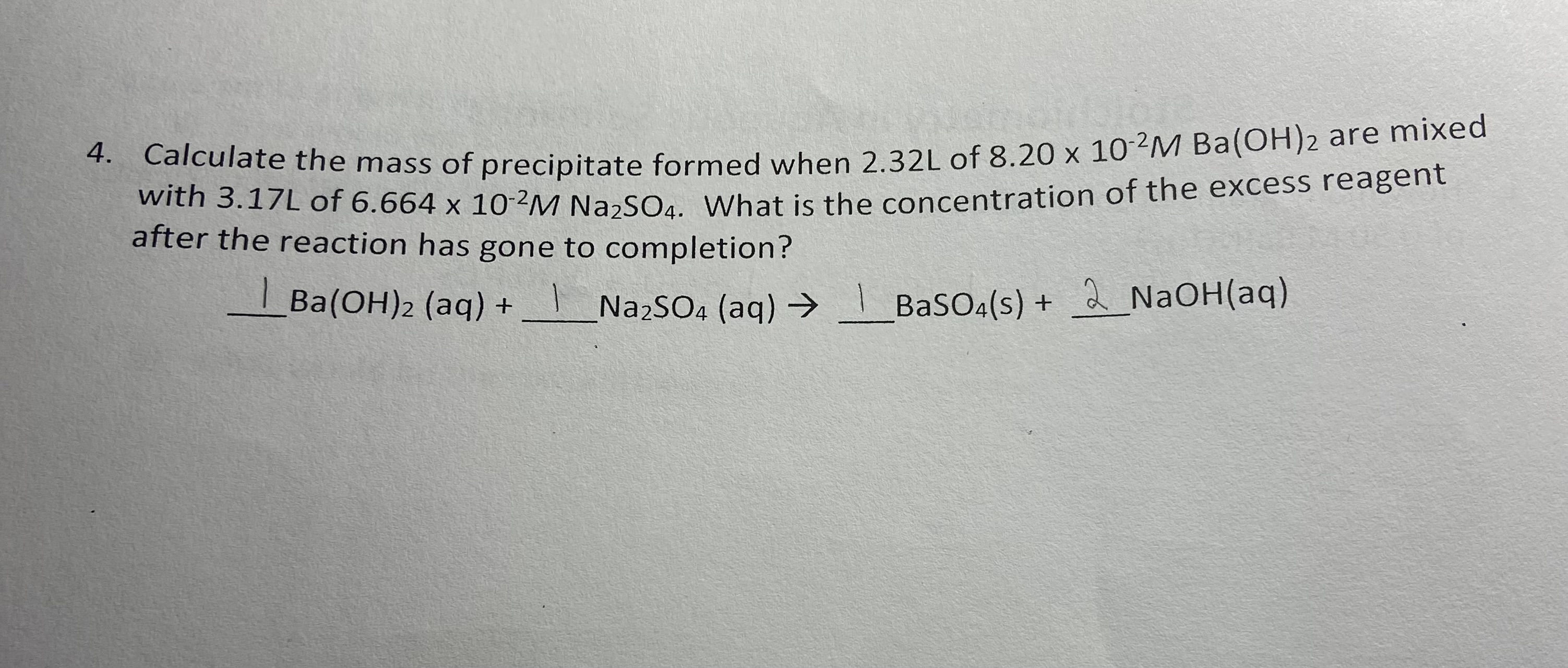Home /
Expert Answers /
Chemistry /
calculate-the-mass-of-precipitate-formed-when-2-32l-of-8-20-x10-2-m-ba-oh-2-are-mixed-with-3-17-pa609
(Solved): Calculate the mass of precipitate formed when 2.32L of 8.20 x10^(-2)M Ba(OH)_(2) are mixed with 3.17 ...
Calculate the mass of precipitate formed when 2.32L of 8.20 x10^(-2)M Ba(OH)_(2) are mixed with 3.17L of 6.664 x10^(-2)M Na_(2)SO_(4). What is the concentration of the excess reagent after the reaction has gone to completion?
4. Calculate the mass of precipitate formed when of are mixed with of . What is the concentration of the excess reagent after the reaction has gone to completion?