Home /
Expert Answers /
Chemistry /
calculate-the-mass-in-grams-of-precipitate-formed-when-2-27-mathrm-l-of-0-0820-math-pa973
(Solved): Calculate the mass (in grams) of precipitate formed when \( 2.27 \mathrm{~L} \) of \( 0.0820 \math ...
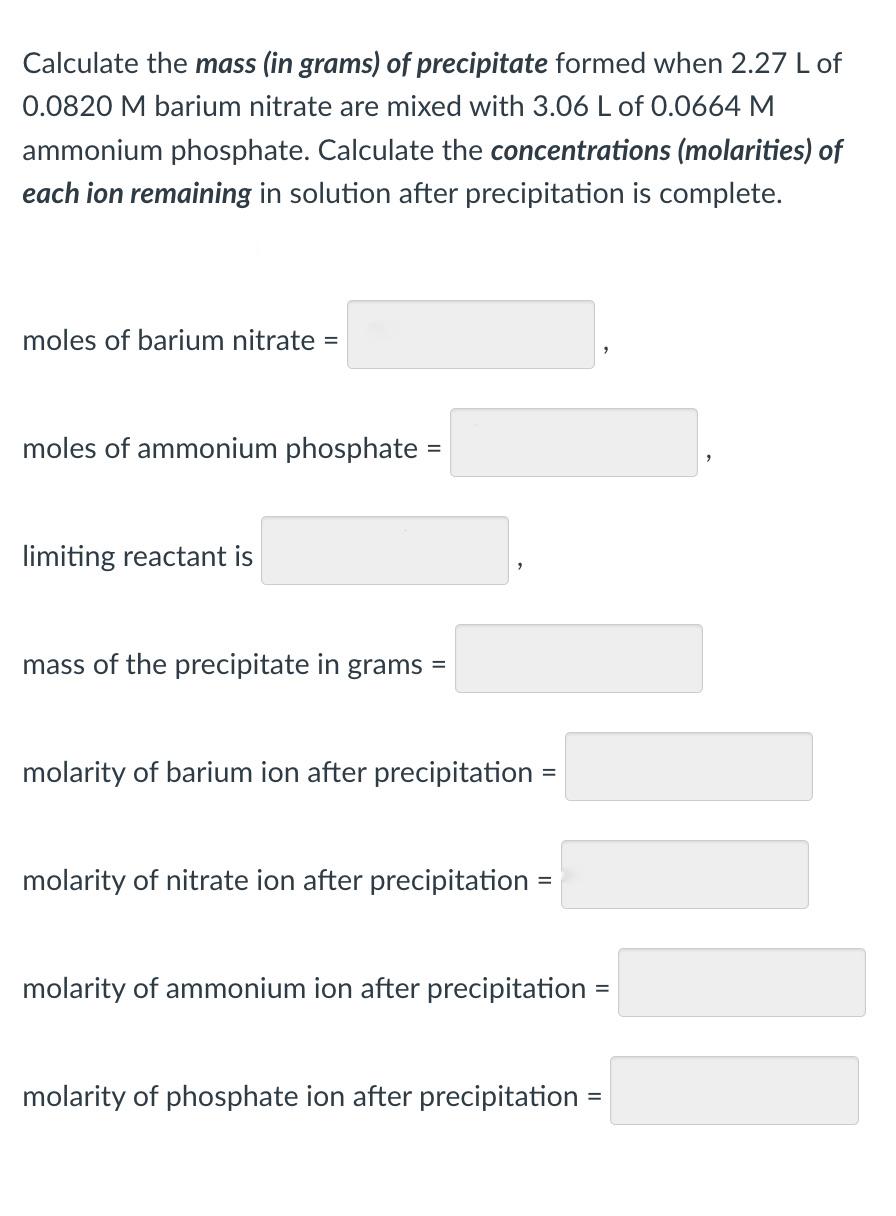
Calculate the mass (in grams) of precipitate formed when \( 2.27 \mathrm{~L} \) of \( 0.0820 \mathrm{M} \) barium nitrate are mixed with \( 3.06 \mathrm{~L} \) of \( 0.0664 \mathrm{M} \) ammonium phosphate. Calculate the concentrations (molarities) of each ion remaining in solution after precipitation is complete. moles of barium nitrate \( = \) moles of ammonium phosphate \( = \) limiting reactant is mass of the precipitate in grams \( = \) molarity of barium ion after precipitation = molarity of nitrate ion after precipitation = molarity of ammonium ion after precipitation \( = \) molarity of phosphate ion after precipitation \( = \)
Expert Answer
Molar mass of Barium nitrate = 261.337 g/mol Molar mass of ammonium sulphate = 149.08 g/mol Molar mass of Barium phosphate = 601.93 g/mol Molarity = number of moles/ Volume in liter Number of mole = molarity × volume in liter Number of mole of Barium