Home /
Expert Answers /
Chemistry /
calculate-the-free-energy-change-of-hydrogen-ions-across-the-mitochondrial-membrane-if-the-concent-pa290
(Solved): Calculate the free energy change of hydrogen ions across the mitochondrial membrane if the concent ...
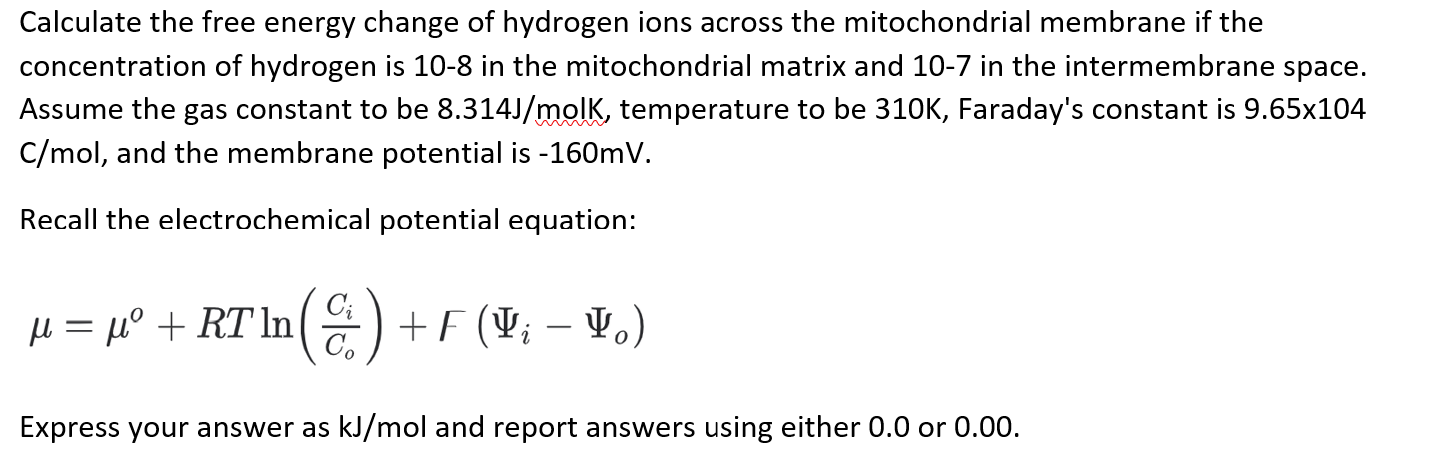
Calculate the free energy change of hydrogen ions across the mitochondrial membrane if the concentration of hydrogen is 10-8 in the mitochondrial matrix and 10-7 in the intermembrane space. Assume the gas constant to be \( 8.314 \mathrm{~J} / \mathrm{molK} \), temperature to be \( 310 \mathrm{~K} \), Faraday's constant is \( 9.65 \times 104 \) \( \mathrm{C} / \mathrm{mol} \), and the membrane potential is \( -160 \mathrm{mV} \). Recall the electrochemical potential equation: \[ \mu=\mu^{o}+R T \ln \left(\frac{C_{i}}{C_{o}}\right)+\digamma\left(\Psi_{i}-\Psi_{o}\right) \] Express your answer as \( \mathrm{kJ} / \mathrm{mol} \) and report answers using either \( 0.0 \) or \( 0.00 \).