Home /
Expert Answers /
Chemical Engineering /
calculate-the-equilibrium-constant-for-the-vapor-phase-hydration-of-ethylene-at-145-circ-ma-pa242
(Solved): Calculate the equilibrium constant for the vapor-phase hydration of ethylene at \( 145^{\circ} \ma ...
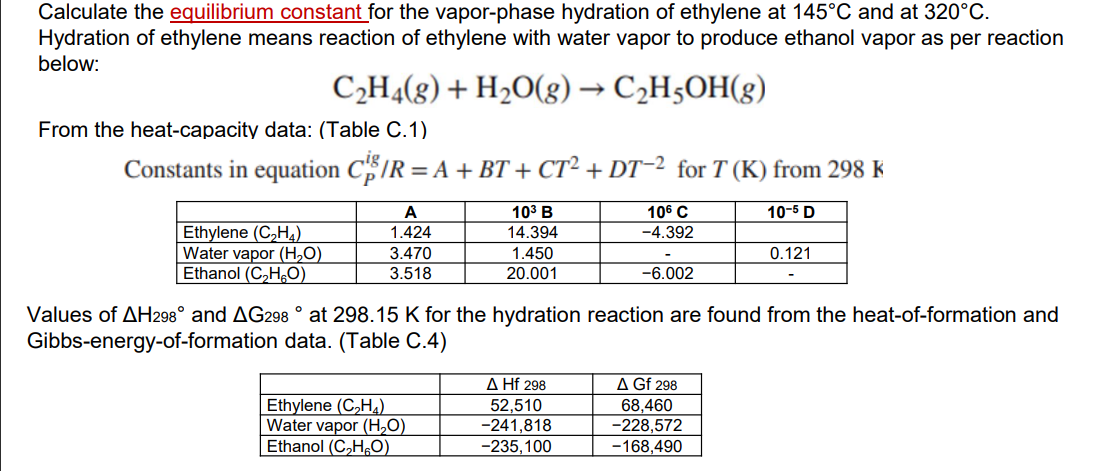
Calculate the equilibrium constant for the vapor-phase hydration of ethylene at \( 145^{\circ} \mathrm{C} \) and at \( 320^{\circ} \mathrm{C} \). Hydration of ethylene means reaction of ethylene with water vapor to produce ethanol vapor as per reaction below: \[ \mathrm{C}_{2} \mathrm{H}_{4}(g)+\mathrm{H}_{2} \mathrm{O}(g) \rightarrow \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}(g) \] From the heat-capacity data: (Table C.1) Constants in equation \( C_{P}^{i g} / R=A+B T+C T^{2}+D T^{-2} \) for \( T(\mathrm{~K}) \) from \( 298 \mathrm{~K} \) Values of \( \Delta \mathrm{H}_{298^{\circ}} \) and \( \Delta \mathrm{G}_{298}{ }^{\circ} \) at \( 298.15 \mathrm{~K} \) for the hydration reaction are found from the heat-of-formation and Gibbs-energy-of-formation data. (Table C.4)
Expert Answer
Given Data C2H4(g) + H2O(g) ? CH3CH2OH(g) At