Home /
Expert Answers /
Chemistry /
calculate-the-concentration-of-hc6h6o6in-an-aqueous-solution-of-0-0631m-ascorbic-acid-pa797
Expert Answer
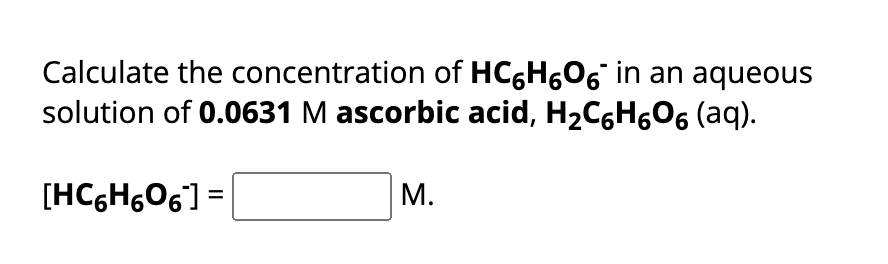
Ka of Ascorbic acid is Given that the Molarity of Ascorbic acid is 0.0631M We need to find the Concentration of