Home /
Expert Answers /
Chemistry /
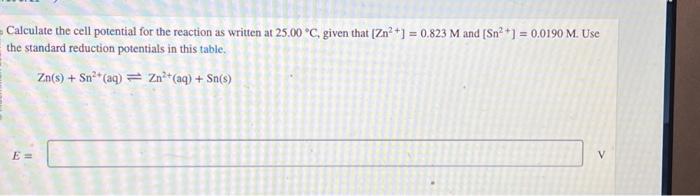
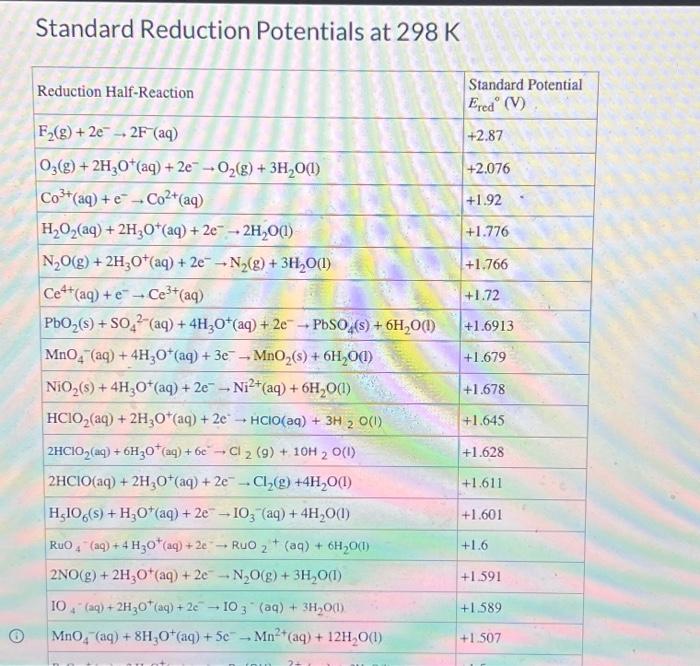
calculate-the-cell-potential-for-the-reaction-as-written-at-25-00c-given-that-zn2-0-823m-an-pa305
Expert Answer
The above question can be solved using the following concept, use the nernst equation and then calcu...